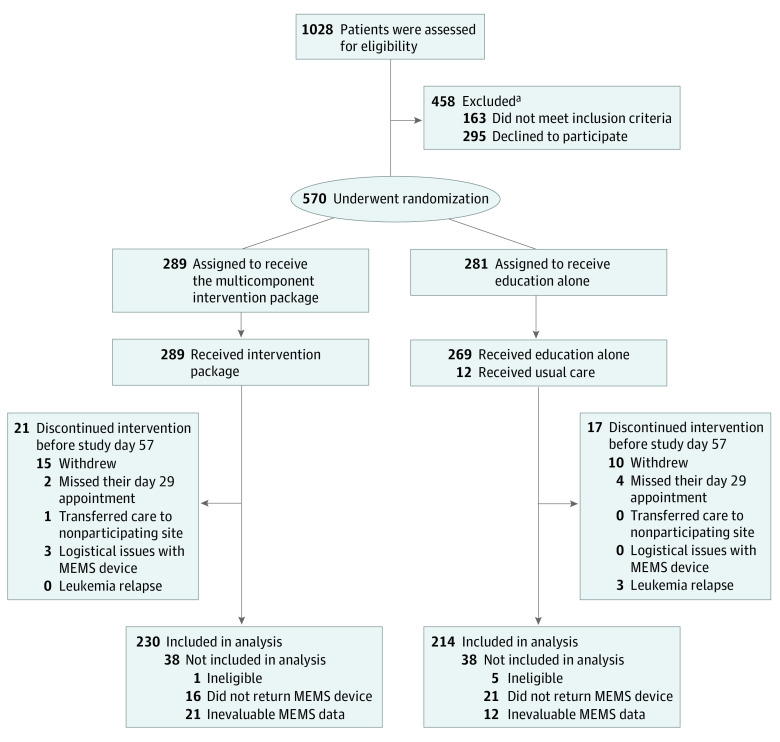
Figure 1. Enrollment and Retention of Patients by Study Group.
Day 29 and day 57 represent days during the trial when the patient returned for a scheduled clinic visit.
aAmong the 163 patients who did not meet the inclusion criteria, 111 were using a pillbox, 20 did not want to receive text message reminders, 12 did not want to use the Medication Event Monitoring System (MEMS) device, 5 did not have a designated caregiver, and 15 had other reasons. Among the 295 patients who declined participation, 114 were not interested, 62 had another reminder system, 24 said they already remembered, 23 said they were too busy or the timing was bad, 3 had physician refusal, and the other 69 had other or no reasons. Lack of access to a text-capable device was not a confounding feature, because for patients who did not have access to a cellular telephone with texting capabilities, we issued a study telephone with unlimited data for the entire study period.