Figure 8.
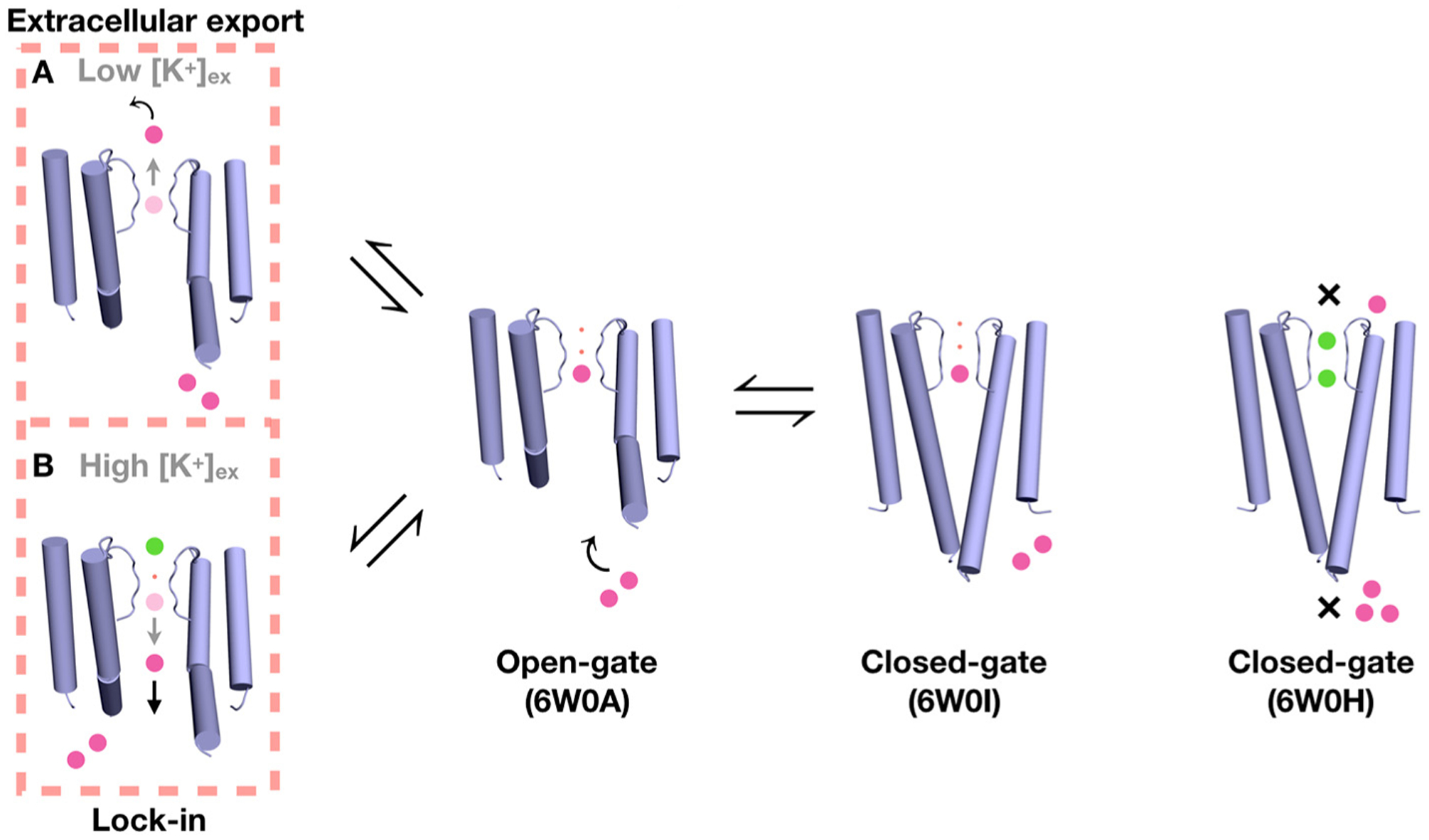
Proposed mechanism for Ba2+ blockade based on the crystal structures, MD simulations, and available functional data [3]. The movement of Ba2+ (magenta sphere) to bind to a closed-conductive KcsA channel (6W0H) by soaking is opposed by the closed inner gate and by the high free-energy barrier from the selectivity filter, (indicated by the ×). No bound Ba2+ is detected in the structure even though bound K+ (green sphere) are observed. Ba2+ can bind to the site S4 of the open-constricted KcsA channel (6W0A), presumably accessing this location via the intracellular side. It can also bind to S4 of a closed-constricted KcsA channel (6W0I) via co-crystalization. The presence of a bound Ba2+ blocks the ionic flow of K+. Two possible scenarios of Ba2+ permeation through the blocked KcsA channel are illustrated (on the left); Ba2+ can be either exported to the extracellular bulk by transiently binding to S2 and S1 (route A), or it can fall back to the intracellular side when locked-in by the binding of an K+ ion from the external side (route B). The small red spheres represent water molecules.
