Figure 1.
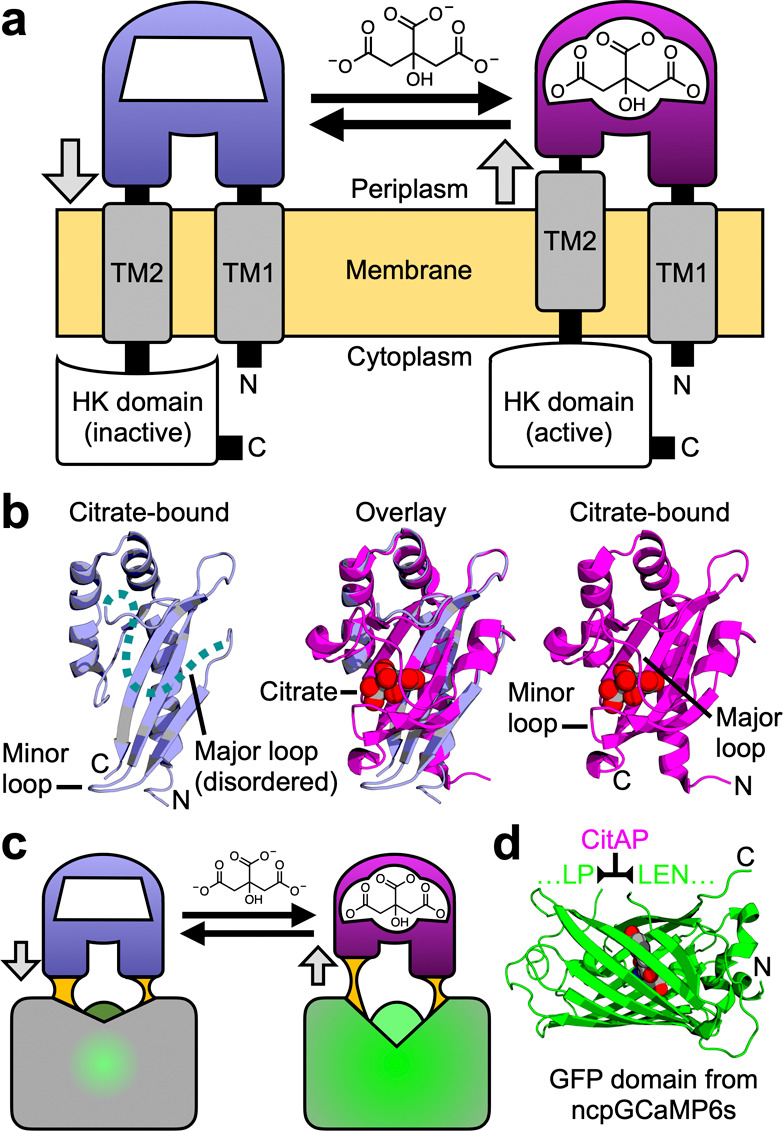
Rationale for the design of a single-FP-based citrate biosensor. (a) Schematic representation of Klebsiella pneumoniae SHK CitA, which is composed of a periplasmic citrate-binding domain (CitAP; light blue, unbound; magenta, bound), connected to transmembrane helices at both its N- (transmembrane helix 1, TM1) and C-termini (transmembrane helix 2, TM2). TM2 is, in turn, connected to an intracellular HK catalytic domain.26 (b) The structures of citrate-free CitAP (left; light blue; PDB ID 2V9A),25 citrate-bound CitAP (right; magenta; PDB ID 2J80),25 and a superposition of the citrate-free and -bound structures (middle). (c) We hypothesized that the piston-type conformational motion at the CitAP termini could be communicated to GFP to allosterically control the chromophore environment and its fluorescent brightness. In this way, the CitAP domain could serve as the basis of construction of a genetically encoded citrate biosensor. (d) To realize this biosensor design, we inserted CitAP into GFP by replacing the CaM-RS20 domain of ncpGCaMP6s27 with CitAP.
