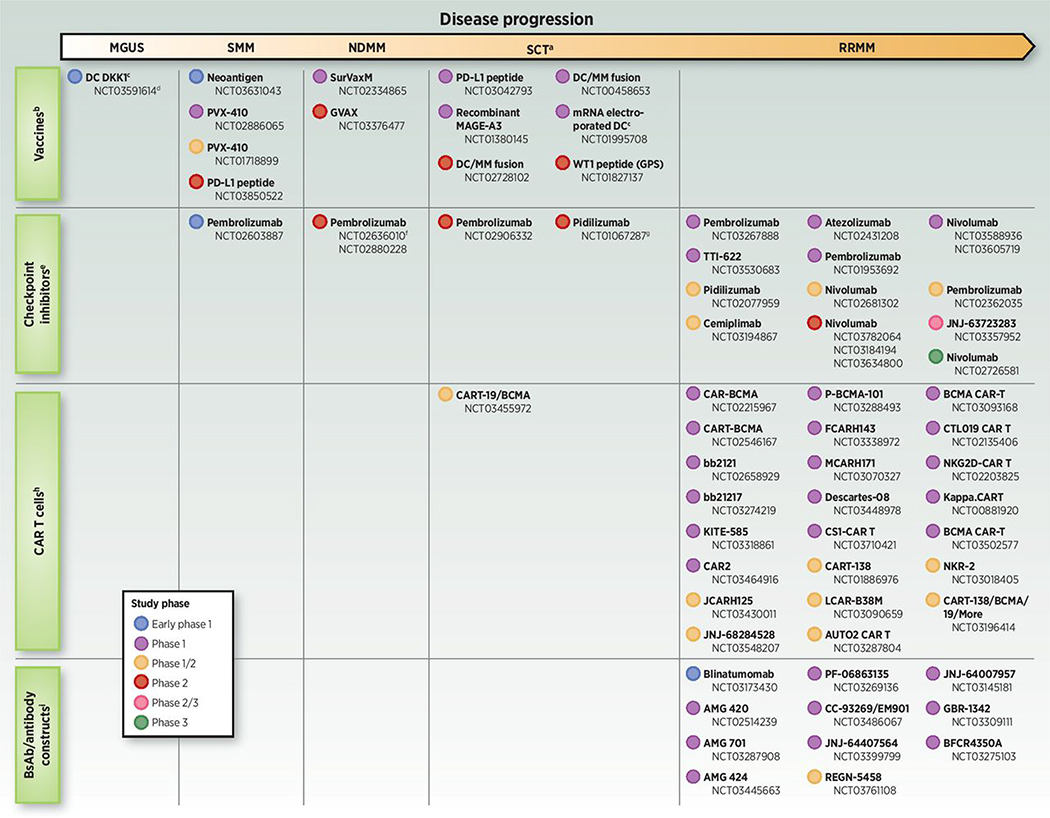
Select T-cell–dependent immuno-oncology studies across the multiple myeloma disease continuum. Studies include early stages of disease (MGUS, SMM, and NDMM), patients with multiple myeloma and low tumor burden (transplant setting), and patients in the relapsed and/or refractory multiple myeloma setting. BCMA, B-cell maturation antigen; bsAb, bispecific antibody; CAR T, chimeric antigen receptor T cell; CPI, checkpoint inhibitor; DC, dendritic cell; GPS, galinpepimut-S; MGUS, monoclonal gammopathy of undetermined significance; MM, multiple myeloma; NDMM, newly diagnosed multiple myeloma; RRMM, relapsed and/or refractory multiple myeloma; SCT, stem cell transplantation; SMM, smoldering multiple myeloma. Based on www.clinicaltrials.gov search conducted on February 20, 2019. aSCT studies may include NDMM or RRMM patients (i.e., not mutually exclusive). bStudies in RRMM are not shown, as the focus of more recent vaccine therapy trials has been for disease stages with lower tumor burden/immunosuppression. cAutologous DC vaccine. dStudy also includes SMM and multiple myeloma patients. eStudies evaluating pembrolizumab/immunomodulatory drug combinations in RRMM are not included due to clinical holds placed on some of these studies. fStudy also includes patients at first relapse. gIn conjunction with DC/multiple myeloma vaccine. hFollowing the completion of our clinicaltrials.gov search, a phase I/II study of CT053, a BCMA-targeting CAR T-cell therapy, has opened (NCT03975907). iFollowing the completion of our clinicaltrials.gov search, a phase I study of TNB-383B, a BCMA-targeting bsAb/antibody construct, has entered clinical trials (NCT03933735).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.