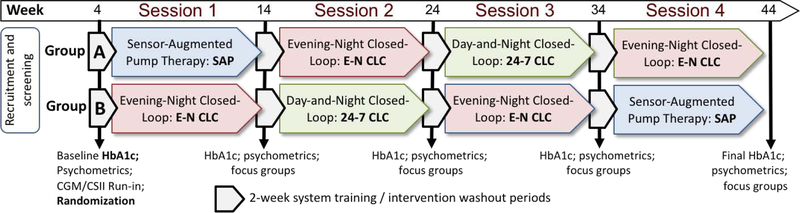
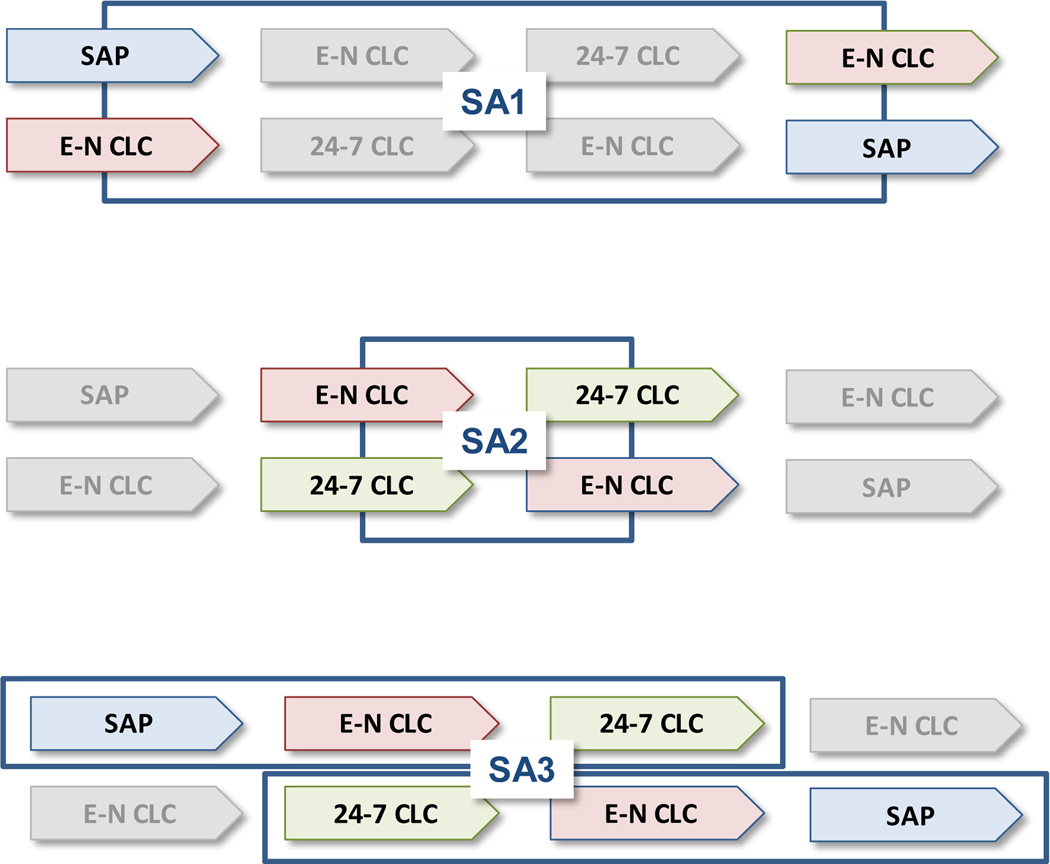
Figure 1.
Study design
Panel 1: Overall Design
Panel 1: Study participants were randomized into one of two groups: Group A following the sequence of sessions SAP → E-N CLC → 24–7 CLC → E-N CLC, and Group B following the sequence of sessions EN CLC → 24–7 CLC → E-N CLC → SAP. Each treatment session continued for 8 weeks; treatment sessions were separated by 2-week washout periods during which HbA1c was measured and questionnaires were collected.
Panel 2: Sub-analyses (SAs) addressing the study objectives:
Panel 2: The overall design include two cross-over designs: SA1 comparing SAP vs. Evening-Night Closed-Loop Control and SAP during the day, and SA2 comparing Evening-Night Closed-Loop Control and SAP during the day vs 24–7 closed-loop control. A third sub-analysis, SA3, allowed the comparison of escalating vs. de-escalting treatments, SAP → E-N CLC → 24–7 CLC (Group A) vs. 24–7 CLC → E-N CLC → SAP (Group B).