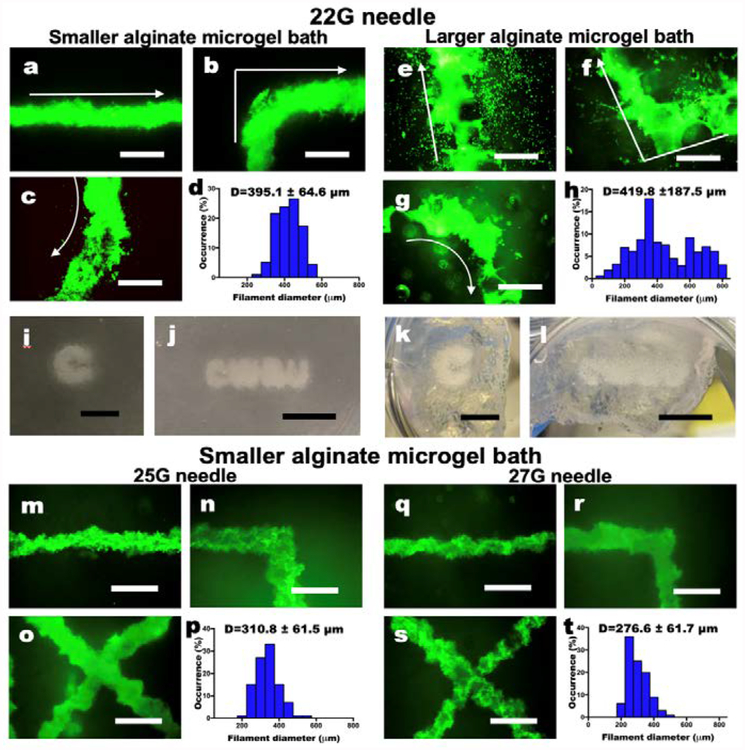
Fig. 3. Characterization of living cell-only bioink.
(a-c) Live/Dead staining of 3D hMSC filaments bioprinted in a straight line, a corner and a curve with a 22 G needle and (d) their diameter distribution in the smaller alginate microgel supporting medium. (e-g) Live/Dead staining of 3D hMSC filaments bioprinted in various configurations with a 22 G needle and (h) their diameter distribution in the larger alginate microgel supporting medium. Arrows indicate the direction of movement of the printing nozzle. Scale bars indicate 600 μm. The Live/Dead images demonstrate high cell viability. Smaller alginate microgels lead to higher resolution printing by limiting diffusion of cells into the pores of the microgel bath. Thickness of the cell filaments also are more narrowly distributed in smaller microgel medium. Images of letters ‘C’ and “CWRU” in (i and j) the smaller and (k and l) larger alginate microgel supporting medium after photocrosslinking. Scale bars indicate 5 mm. (m-o) Live/Dead staining of 3D hMSC filaments bioprinted in various configurations with a 25 G needle and (p) their diameter distribution in the smaller alginate microgel supporting medium. (q-s) Live/Dead staining of 3D hMSC filaments bioprinted in various configurations with a 27 G needle and (h) their diameter distribution in the smaller alginate microgel supporting medium. Scale bars indicate 600 μm. Smaller diameter needles lead to higher resolution printing of the cell filaments, which also are more narrowly distributed. Scale bars indicate 600 μm.