Figure 2: Neuroprotection in dopaminergic neurons.
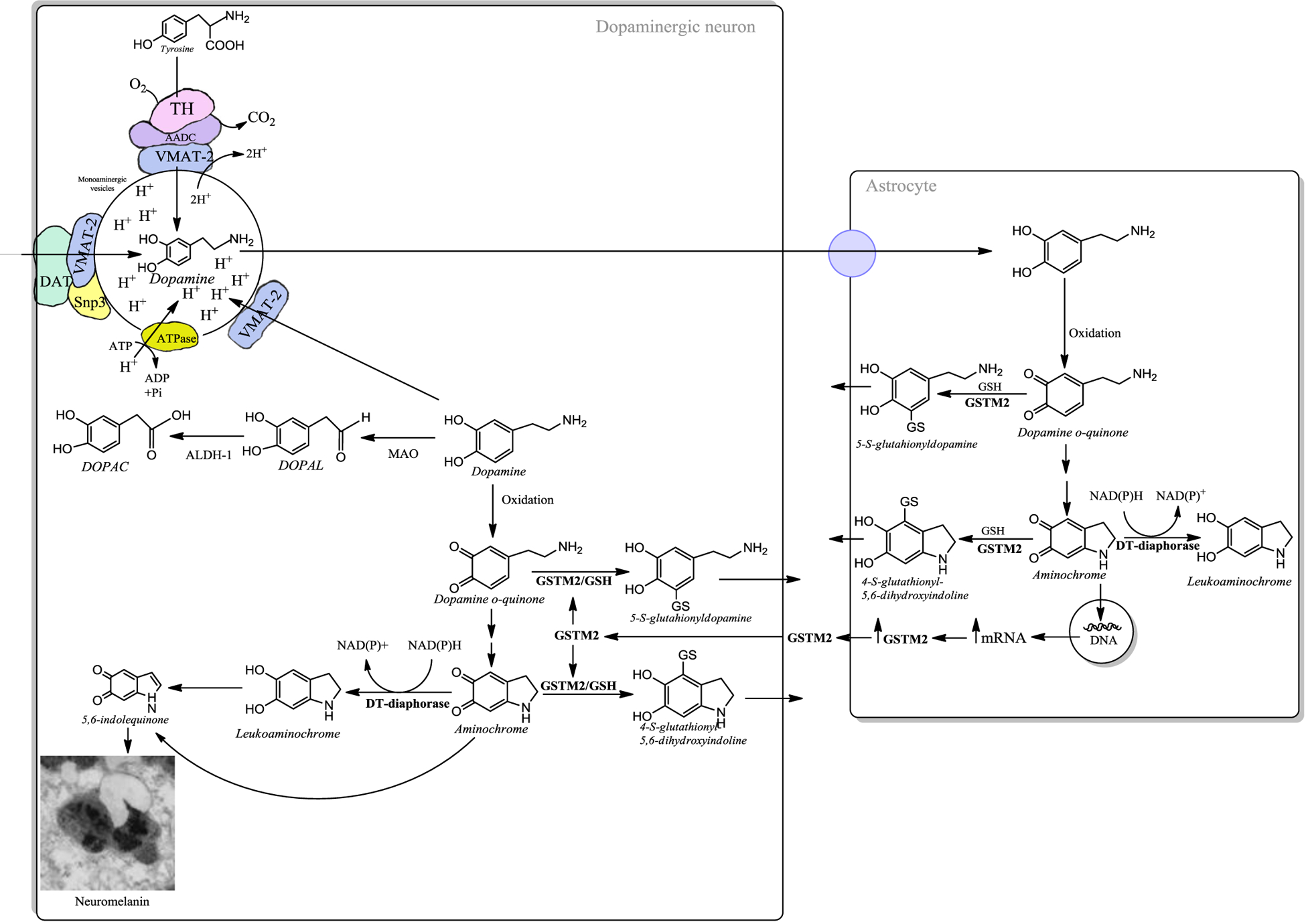
The presence of free dopamine in the cytosol is a risk, because during the oxidation of dopamine to neuromelanin (through a multi-step biosynthetic mechanism that involves iron-catalyzed oxidation, polymerization and reaction with proteins and lipids), or in its degradation catalyzed by monoamine oxidase (MAO in the Fig.), neurotoxic o-quinones can be generated. Therefore, VMAT-2 is the first line of neuroprotection against the neurotoxic effects of dopamine oxidation. VMAT-2 protects dopaminergic neurons by transporting dopamine towards the monoaminergic neurotransmission vesicles. Dopaminergic neurons are likely exposed to dopamine-derived damage when levels of VMAT-2 are not high enough to limit free dopamine and its autoxidation occurs in the cytosol. In this case neuromelanin biosynthesis provides an additional means to limit dopamine toxicity through a neuroprotective mechanism. There are two enzymes that prevent the neurotoxic effects of dopamine o-quinone and aminochrome in dopaminergic neurons. The enzyme DT-diaphorase catalyzes the two-electron reduction of aminochrome to leukoaminochrome, then leading to formation of neuromelanin precursors and the enzyme GSTM2, which can inactivate both dopamine o-quinone and aminochrome by conjugating these o-quinones with glutathione. GSTM2 is expressed only in human astrocytes, which secrete this enzyme. Dopaminergic neurons are able to internalize GSTM2 into the cytosol, where this enzyme conjugates these o-quinones with glutathione which undergo further degradation forming other probable precursors of neuromelanin pigment.
