Figure 3.
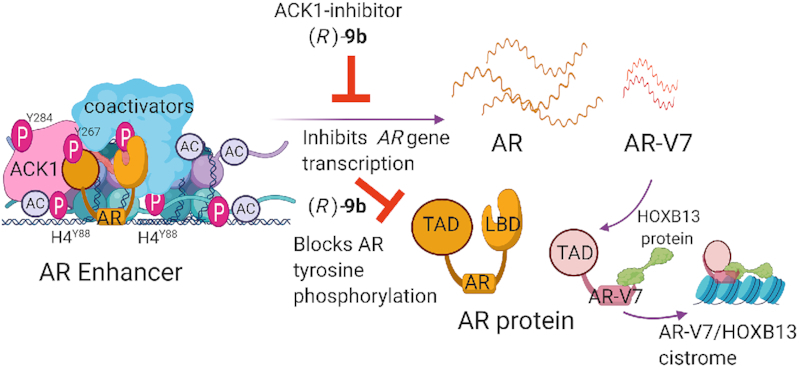
Epigenetic regulation of AR by ACK1 in mCRPCs. The ACK1–AR complex is recruited to the AR enhancer, where it phosphorylates histone H4 at residue Y88, thus driving AR transcription. The ACK1 catalytic inhibitor (R)-9b blocks tyrosine phosphorylation and autoactivation of ACK1 as well as tyrosine phosphorylation of its substrates, thereby inhibiting the androgen-independent AR program in CRPCs. PC cells invariably develop resistance to AR antagonists, and intriguingly, perturbations in AR are common in patients with CRPC. These resistance-causing perturbations include structural alterations in AR enhancers, AR gene amplifications or mutations and AR splice variants that lack the LBD, such as AR-V7. Post-translational modifications that facilitate androgen-independent AR recruitment to chromatin regions distinct from those targeted by androgen-bound AR, as well as the interaction of AR-V7 with HOXB13, are among the distinct mechanisms of transcriptional regulation in CRPCs. Figure was created with www.biorender.com.
