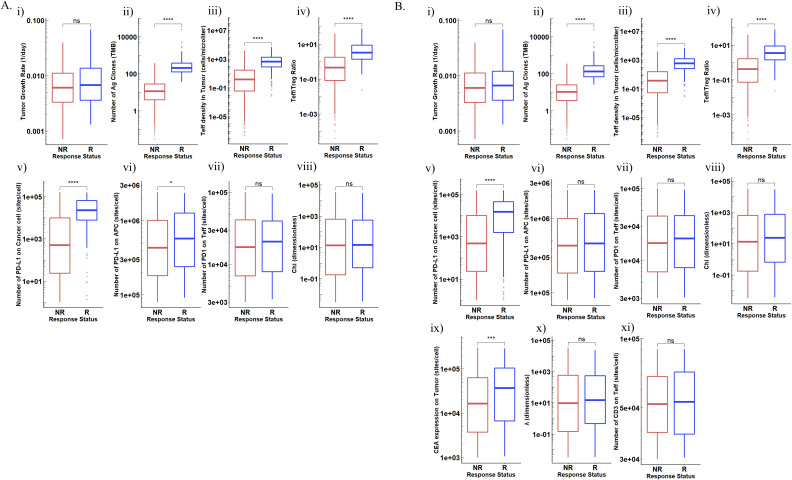
Figure 4.
Distributions of potential biomarkers in NR and R in (A). Atezolizumab monotherapy (B). Combination therapy. (i) Tumor growth rate; (ii) TMB; (iii) ieff density in tumor; (iv) Teff/Treg ratio in tumor; (v) PD-L1 expression in cancer cells; (vi). PD-L1 expression in APCs; (vii) PD-1 expression in teff; (viii) Cross-arm binding efficiency χ of atezolizumab; (ix) CEA expression in cancer cells; (x) Cross-arm binding efficiency λ of cibisatamab; (xi) CD3 expression in teff; (xii) CD3-cibisatamab binding affinity. APCs, antigen-presenting cells; CEA, carcinoembryonic antigen; NS, not significant; PD1, programmed cell death protein 1; PD-L1, PD-ligand 1; TMB, tumor mutational burden. * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001, **** P ≤ 0.0001.