Abstract
Purpose
There are increasing reports in the literature of high rates of coagulopathy and venous thromboembolism (VTE) among hospitalized patients with coronavirus disease 2019 (COVID-19). Understanding of these abnormalities is continually evolving, but these conditions may pose a risk to COVID-19 patients beyond the risk typically seen in critically ill patients.
Summary
There are currently no widely accepted evidence-based guidelines regarding specifics related to treatment and prevention of COVID-19–related coagulopathies. Areas of management requiring clinical equipoise include agent selection and dosing, continuation vs interruption of home oral anticoagulant therapy during hospital admission, and postdischarge VTE prophylaxis. Clinicians may wish to consider use of a stratified, 3-tiered approach of low-intensity anticoagulation, intermediate-intensity anticoagulation, and therapeutic-dose anticoagulation. Patients can be categorized by tier depending on their risk factors for VTE, acuity of illness, and laboratory values such as D-dimer level.
Conclusion
Practical guidance on anticoagulation considerations and dosing suggestions are provided to assist clinicians faced with challenging anticoagulation-related situations in caring for hospitalized COVID patients until formal evidence-based guidelines become available.
Keywords: anticoagulation, COVID-19, deep vein thromboprophylaxis, heparin, low molecular weight heparin, venous thromboembolism
KEY POINTS.
COVID-19 is a hypercoagulable state, and many patients with COVID-19 may experience venous thromboembolism (VTE) despite standard VTE prophylaxis.
Evidence from randomized controlled trials to guide decisions regarding anticoagulation in patients with COVID-19 is currently lacking.
This article offers practical guidance based on current experiential evidence in real-world settings to assist clinicians in managing anticoagulation in patients with COVID-19.
There are increasing reports in the literature of high rates of coagulopathy and venous thromboembolism (VTE) among hospitalized patients with coronavirus disease 2019 (COVID-19). 1-5 Hematologic abnormalities commonly seen in patients with COVID-19 include elevations in D-dimer and fibrinogen, prolonged prothrombin time and activated partial thromboplastin time (aPTT), and changes in levels of inflammatory markers such as C-reactive protein (CRP), interleukin-6, and ferritin. Understanding of these abnormalities is continually evolving, but they may pose a risk to patients with COVID-19 beyond the risk typically seen in critically ill patients. Tang et al1 reported that abnormalities in coagulation markers are indicators of disease severity and that higher D-dimer levels are associated with increased mortality. A retrospective analysis of 81 patients with severe COVID-19 found that those experiencing VTE were more likely to be older and to have prolonged aPTT and elevated D-dimer levels.2 Furthermore, the incidence of thrombotic complications has been reported to be 20% to 31% in critically ill patients with COVID-19 despite use of standard VTE prophylaxis.3,4 Poissy et al5 reported an increased incidence of pulmonary embolism (PE) amongst COVID-19 patients admitted to an intensive care unit. Of the 22 patients who developed PE, 20 patients (90%) were receiving VTE prophylaxis.
In an encouraging sign for treatment of patients with COVID-19, available anticoagulants have shown some benefit. A small observational study found that among patients with a D-dimer level greater than 6 times the upper limit of normal (ULN), those treated with heparin had 28-day mortality approximately 20 percentage points lower than those not treated with heparin (32.8% vs 52.4%; P = 0.017).6 In an additional observational study of patients hospitalized for COVID-19 in New York City, promising results were reported for systemic, treatment-dose anticoagulation in mechanically ventilated patients; those who received treatment-dose anticoagulation experienced in-hospital mortality of 29.1%, compared with a death rate of 62.7% among those who did not.7
Although some helpful guidance has been published recently, there are currently no widely accepted evidence-based guidelines available regarding specifics related to treatment and prevention of COVID-19–related coagulopathies.8-12 Areas of current clinical uncertainty include dose and agent of choice, continuation vs interruption of home oral anticoagulant therapy while admitted, and postdischarge VTE prophylaxis. Additional challenges are found in use of VTE risk stratification tools that do not incorporate COVID-19 as a risk factor.13-16 This article discusses practical considerations in anticoagulation management in the context of COVID-19 to assist clinicians until more formal evidence-based guidelines become available.
General treatment considerations
Guidance from the International Society on Thrombosis and Haemostasis recommends prophylactic anticoagulation for all patients who require hospital admission for COVID-19 in the absence of any contraindications such as active bleeding and a platelet count less than 250 × 103/µL.9 For critically ill patients with COVID-19, barriers to obtaining diagnostic testing to confirm or rule out active bleeding, such as endoscopy or colonoscopy, may be present, further complicating treatment decisions. Low-molecular-weight heparin (LMWH) has been suggested as the anticoagulant of choice (in preference to unfractionated heparin [UH]) for reducing healthcare staff contact with patients in the absence of a compelling reason not to use it.9 Fondaparinux may be considered for patients with a history of heparin-induced thrombocytopenia or aversion to receipt of porcine derivatives.17 There is some evidence to suggest heparins have anti-inflammatory properties18,19; theoretically, this may have added benefit in COVID-19 cases where proinflammatory cytokines are markedly elevated. However, clinicians may wish to consider patient-specific factors in order to guide their decisions. These factors include but are not limited to concurrent comorbidities, relevant laboratory values, acuity, mobility, weight, renal function, bleeding risk, and need for invasive procedures or interventions.
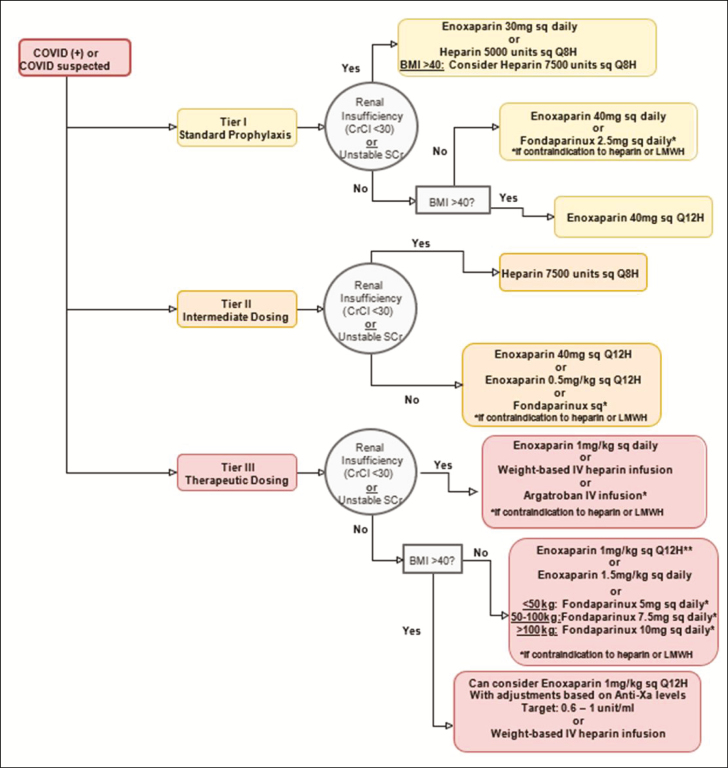
Upon review of the available evidence, we believe that clinicians may wish to consider a 3-tiered approach to stratifying anticoagulation intensity (Figure 1), with consideration of the aforementioned factors to guide and assist in decision making. The 3 tiers are low-intensity anticoagulation (tier I), intermediate-intensity anticoagulation (tier II), and therapeutic-dose anticoagulation (tier III). We recognize this tiered approach is not universally accepted; however, there is increasing evidence that many patients may develop VTE despite receipt of standard VTE prophylaxis. Further, not all patients require therapeutic anticoagulation; therefore, tier II anticoagulation has been suggested as an option.3,20
Figure 1.
Flowchart of 3-tiered approach to stratification of anticoagulation intensity. Anti-Xa indicates anti–factor Xa; BMI, body mass index; CrCl, creatinine clearance; IV, intravenous; LMWH, low-molecular-weight heparin; SCr, serum creatinine; sq, subcutaneously.
An additional area of clinical controversy involves patients’ use of home oral anticoagulants while admitted to the inpatient setting. Hospitalized patients, particularly critically ill patients, may require invasive procedures, which may require interruption of oral anticoagulation therapy. For any patient who is already taking an oral anticoagulation at home, it may be prudent to transition to therapeutic-dose LMWH or parenteral UFH infusion for the duration of hospitalization unless contraindications exist.10 Further, there is little published data regarding the use of oral anticoagulants in the COVID-19 patient population. In the setting of renal dysfunction, use of intravenous UFH along with anti–factor Xa (anti-Xa) monitoring may be preferred.
Patient selection for tiered anticoagulation therapy
Tier I.
Patients with COVID-19 who may be appropriate candidates for tier I anticoagulation include those with indications for standard prophylaxis. This tier should be considered for patients without known thrombi or known malignancy; these are generally lower-acuity patients, such as non–critically ill medical patients. This approach may be considered for patients with D-dimer levels less than 3 times the ULN that are not trending upward.
Tier II.
Tier II, or intermediate-intensity, anticoagulation may be appropriate for a patient with COVID-19 who requires a higher-intensity standard prophylaxis regimen. A patient may be categorized into tier II based on acuity and/or VTE risk factors (eg, a patient who is on a general medical floor but clinically deteriorating, with an upward trend in inflammatory marker and/or D-dimer levels). Tier II therapy could be considered for patients whose D-dimer level is above 3 times the ULN. To determine VTE risk factors that may place patients in this category, clinicians may wish to consider risk stratification models such as the Caprini score, IMPROVE risk score, and Padua Prediction Score models, all of which have been used in acutely ill hospitalized patients.13-16 VTE risk factors considered when using the Padua Prediction Score model are listed in Table 1. Additionally, all critically ill patients should be considered for tier II therapy, at a minimum, if they do not have risk factors for VTE and/or the clinician team is otherwise not concerned about VTE development. Klok et al3 and Connors et al20 showed that VTE incidence was higher in critically ill patients with COVID-19 than in other critically ill patients despite their receipt of VTE prophylaxis; therefore, it is not unreasonable to consider intensified VTE prophylaxis in critically ill patients with COVID-19.
Table 1.
Risk Factors for Venous Thromboembolism13,a
| Factor(s) | Score |
|---|---|
| Active cancerb | 3 |
| Previous VTE (superficial vein thrombosis excluded) | 3 |
| Reduced mobilityc | 3 |
| Already known thrombophilic conditiond | 3 |
| Recent trauma and/or surgery (≤1 month) | 2 |
| Elderly age (>70 years) | 1 |
| Heart and/or respiratory failure | 1 |
| Acute myocardial infarction or ischemic stroke | 1 |
| Acute infection and/or rheumatologic disorder | 1 |
| Obesity (BMI ≥30) | 1 |
| Ongoing hormonal treatment | 1 |
| Total score | ≥4 = high VTE risk |
Abbreviations: BMI, body mass index; VTE, venous thromboembolism.
aAdapted from Padua Prediction Score.13
bPatients who have local or distant metastases and/or have received chemotherapy or radiotherapy in the previous 6 months.
cBedrest with bathroom privileges (due to either patient’s limitations or physician’s orders) for at least 3 days.
dDefects of antithrombin, protein C or S, or factor V Leiden; G20210A prothrombin mutation; or antiphospholipid syndrome.
Tier III.
Tier III therapy may be chosen for those patients with COVID-19 who have known or strongly suspected VTE. Patients presenting with acute coronary syndrome should be categorized into this tier.9 This tier can strongly be considered for any patient with a D-dimer level greater than 6 times the ULN or in a continued upward trend. Moreover, therapeutic anticoagulation should be strongly considered for any patient with clinical sequelae of possible VTE, including an otherwise unexplained increase in oxygen requirement (in the absence of another obvious cause, such as worsening radiographic evidence of acute respiratory distress syndrome), a need for mechanical ventilation, alveolar dead space (ie, the area occupied by alveoli that do not participate in gas exchange), and organ failure (eg, acute kidney injury) with concern for microvascular thrombi.2
Agent selection for tiered anticoagulation therapy
Tier I.
For low-intensity anticoagulation, clinicians may wish to consider LMWH at standard prophylactic doses. For example, enoxaparin 40 subcutaneously once daily. For obese patients with a body mass index (BMI) greater than 40, an increase in LMWH dose may be considered. Based upon bariatric data available, enoxaparin 40 mg subcutaneously every 12 hours may be employed for standard VTE prophylaxis.21 In the presence of renal insufficiency, LWMH can be renally dose-adjusted (for example, enoxaparin 30 mg subcutaneously daily) or subcutaneous UFH therapy can be used instead. Preference may be given to subcutaneous UFH when renal function is poor or labile. Increased doses of UFH (eg, heparin 7,500 units subcutaneously every 8 hours) may be an option for obese patients with a BMI of >40 in the setting of renal insufficiency.22,23 Monitoring of anti-Xa levels during use of LMWH is not generally recommended; however, assaying anti-Xa levels to target a goal anti-Xa value of 0.2 to 0.5 unit/mL may be considered for obese patients.24 Blood sampling for determination of the anti-Xa level should occur approximately 4 hours after a subcutaneous dose is administered and (to ensure a steady-state concentration) after approximately 4 doses. In the event the level falls outside the target range, it is recommended to exercise best clinical judgment in adjusting doses. In cases where heparin may be contraindicated and renal function allows, fondaparinux administered at a dosage of 2.5 mg subcutaneously daily may be considered for VTE prophylaxis.25
Tier II.
For patients in whom intermediate-intensity anticoagulation is appropriate, employing a higher-than-standard dose may be considered under the assumption that it may confer additional benefit in VTE prevention. Enoxaparin 40 mg subcutaneously every 12 hours may be an option for patients with a BMI of <40. Alternatively, a regimen of enoxaparin 0.5 mg/kg subcutaneously every 12 hours may be considered, especially if the patient is obese.5,26 In the setting of renal insufficiency, heparin can be used at a higher dose (7,500 units subcutaneously every 8 hours).22 Should a patient categorized into this tier have a change in clinical status, such as increased oxygen requirements, clinical evaluation for VTE with either point-of-care ultrasound or formal venous Doppler studies should be pursued if available. Until VTE can be ruled out, VTE therapeutic-dose anticoagulation should be strongly considered. Currently available data are not sufficient to support employment of therapeutic-dose prophylaxis for patients in this tier.6,7
Tier III.
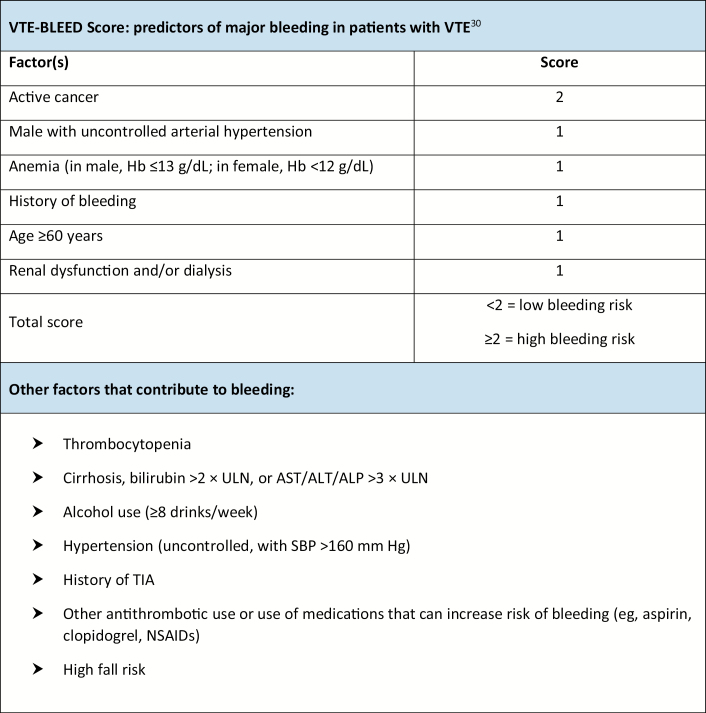
Recommendations for high-intensity anticoagulation in critically ill patients with COVID-19 call for use of a therapeutic dosage of LMWH, such as enoxaparin 1 mg/kg subcutaneously every 12 hours (for patients with creatinine clearance [CLcr] above 30 mL/min) or 1 mg/kg subcutaneously once daily (for those with CLcr of less than 30 mL/min) or intravenous UFH titrated to maintain institution-specific therapeutic levels. Enoxaparin 1.5 mg/kg subcutaneously daily may be considered for a non–critically ill patient (with stable and preserved renal function) in order to reduce the need for patient contact. Monitoring of UFH therapy via anti-Xa assay should be used preferentially if available due to potentially elevated baseline aPTT levels in patients with COVID-19.12 Use of LMWH is advantageous due to a need for less frequent laboratory monitoring and a lower workload burden on nursing staff. In obese patients, clinicians may wish to consider use of enoxaparin at a dosage of 1 mg/kg subcutaneously every 12 hours (for patients with CLcr above 30 mL/min), with monitoring of anti-Xa levels to target a goal anti-Xa value of 0.6 to 1.0 unit/mL.27 Blood samples for anti-Xa level determination should be drawn approximately 4 hours after a subcutaneous dose is administered and after approximately 4 doses. Again, if the anti-Xa level falls outside the target range, clinical judgement is necessary for dose adjustment. UFH may be preferred when bleeding risk is elevated because it has a short half-life and its effects are easily reversible. (Factors to consider in bleeding risk evaluation are listed in Figure 2.) Intravenous UFH may also be an option for patients requiring invasive procedures. For critically ill patients, intravenous UFH may be the preferred agent due to their risk of acute renal dysfunction. Therapeutic heparin should also be considered for all critically ill patients receiving dialysis, including continuous renal replacement therapy (CRRT), and initiated according to institutional CRRT or VTE protocols.10 It is important to ensure communication between pharmacy personnel and the nephrology service in order to balance risks of filter clotting and bleeding in the setting of uremia. In cases involving contraindications to heparin use, clinicians may wish to consider use of therapeutic fondaparinux dosed according to patient weight.17 Intravenous argatroban may be considered in the setting of renal impairment or labile renal function in cases where heparin is contraindicated and fondaparinux may not be ideal.28 At this time, direct thrombin inhibitors should be considered only if there are contraindications to heparin use due to a lack of data specific to the COVID-19 population. It is currently unclear if patients with COVID-19 who have confirmed VTE differ from patients with VTE but without COVID-19, and at this time both groups are being treated similarly with conventional therapeutic anticoagulation.
Figure 2.
Factors to consider in evaluation of bleeding risk in patients with venous thromboembolism. ALP indicates alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hb, hemoglobin; NSAID, nonsteroidal anti-inflammatory drug; SBP, systolic blood pressure; TIA, transient ischemic attack; ULN, upper limit of normal.
Discharge considerations
Post–hospital discharge VTE prophylaxis represents another area of clinical uncertainly that will require a careful weighing of the risks vs potential benefits of ongoing therapy. We suggest that upon discharge all patients, regardless of tier, be assessed for ongoing need for continued prophylaxis. Careful examination of additional risk factors for VTE, such as prolonged immobility while in quarantine, as well as trends in laboratory values, such as D-dimer, CRP, ferritin, and lactate dehydrogenase levels, should be reviewed. Elevated inflammatory markers may indicate an increased risk of VTE upon a patient’s discharge to home, and continued prophylaxis may be warranted. In the absence of randomized, controlled clinical trials, clinicians may wish to consider use of LMWH instead of direct-acting oral anticoagulants (DOACs) or other agents due to the former’s anti-inflammatory effects. DOACs, however, may become an option if compliance concerns arise (eg, a patient’s unwillingness to use parenteral therapy or inability to self-inject). Additionally, patient-specific factors such as insurance and cost considerations must be considered. Duration of prophylactic therapy should be determined on a case-by-case basis, taking into account bleeding risk. Consideration of extended prophylaxis of up to 45 days may be appropriate in patients at increased risk for VTE who are at low risk for bleeding.10 For patients who had been using a DOAC at home and were transitioned to parenteral anticoagulation during an inpatient stay, a transition back to home DOAC therapy may be considered in the absence of evidence of treatment failure.
Upon discharge of patients with confirmed or suspected VTE, continuation of anticoagulation treatment for minimum 3 months for provoked VTE can be chosen. Treatment with therapeutic-dose LMWH or DOAC therapy can be considered in preference to warfarin, in accordance with current guidelines.29 If VTE is not confirmed at time of discharge due to lack of test availability, the clinician may wish to treat according to conventional protocols for VTE. Follow-up with primary care or specialty providers for ongoing reevaluation and extension of therapy must be ensured.
We recognize that the guidance presented in this article has significant limitations, and we hope that clinicians will benefit from a growing body of evidence-based and standardized guidelines on the issues discussed here. As with all treatment options for patients with COVID-19, this is a fluid situation. The preceding is not intended as a practice management protocol; rather, the intent is to provide practical suggestions for clinicians treating this novel disease.
Acknowledgments
The authors wish to acknowledge their employment by as well as the support and resources of Veterans Affairs Western New York Healthcare System and State University of New York at Buffalo School of Pharmacy and Pharmaceutical Sciences. The authors wish to acknowledge Jessica Swiderek and Laura Wilkinson, PharmD, for their contributions to the article manuscript.
Disclosures
The authors have declared no potential conflicts of interest.
References
- 1. Tang N, Li D, Wang X, et al. . Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cui S, Chen S, Li X, et al. . Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klok FA, Kruip M, van der Meer NJM, et al. . Incidence of thrombotic complications in critically ill icu patients with covid-19. Thromb Res. 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Middeldorp S, Coppens M, van Haaps TF, et al. . Incidence of venous thromboembolism in hospitalized patients with COVID-19 [published online April 19, 2020]. J Thromb Haemost. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Poissy J, Goutay J, Caplan M, et al. . Pulmonary embolism in covid-19 patients: Awareness of an increased prevalence [published online ahead of print April 24, 2020]. Circulation. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [Google Scholar]
- 6. Tang N, Bai H, Chen X, et al. . Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paranjpe I, Fuster V, Lala A, et al. . Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19 [published online May 2020]. J Am Coll Cardiol. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bikdeli B, Madhavan MV, Jimenez D, et al. . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up [published online April 17, 2020]. J Am Coll Cardiol. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spyropoulos AC, Levy JH, Ageno W, et al. ; for Subcommittee on Perioperative and Critical Care Thrombosis and Haemostasis of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19 Thrombosis UK website. https://thrombosisuk.org/downloads/ISTH%20VTE%20guidance%20052720%20%20jth.14929.pdf. Accessed May 13, 2020.
- 10. Baumann-Kreuziger L, Lee A, Garcia D, Cuker A,Cushman M, Connors JM. COVID-19 and VTE/anticoagulation: frequently asked questions https://hematology.org/covid-19/covid-19-and-vte-anticoagulation. Accessed May 13, 2020.
- 11. COVID-19 Treatment Guidelines Panel, National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed May 16, 2020. [PubMed]
- 12. Barnes GAB, Allen A, Clark A, et al. . Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbar S, Noventa F, Rossetto V, et al. . A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450-2457. [DOI] [PubMed] [Google Scholar]
- 14. Cronin M, Dengler N, Krauss ES, et al. . Completion of the updated Caprini risk assessment model (2013 version). Clin Appl Thromb Hem. 2019;25:1076029619838052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibson CM, Spyropoulos AC, Cohen AT, et al. . The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1(1):e56-e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spyropoulos AC, Anderson FA Jr, FitzGerald G, et al. . Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706-714. [DOI] [PubMed] [Google Scholar]
- 17. Efird LE, Kockler DR. Fondaparinux for thromboembolic treatment and prophylaxis of heparin-induced thrombocytopenia. Ann Pharmacother. 2006;40(7-8):1383-1387. [DOI] [PubMed] [Google Scholar]
- 18. Downing LJ, Strieter RM, Kadell AM, et al. . Low-dose low-molecular-weight heparin is anti-inflammatory during venous thrombosis. J Vasc Surg. 1998;28(5):848-854. [DOI] [PubMed] [Google Scholar]
- 19. Nasiripour S, Gholami K, Mousavi S, et al. . Comparison of the effects of enoxaparin and heparin on inflammatory biomarkers in patients with ST-segment elevated myocardial infarction: a prospective open label pilot clinical trial. Iran J Pharm Res. 2014;13(2):583-590. [PMC free article] [PubMed] [Google Scholar]
- 20. Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lalama JT, Feeney ME, Vandiver JW, et al. . Assessing an enoxaparin dosing protocol in morbidly obese patients. J Thromb Thrombolysis. 2015;39(4):516-521. [DOI] [PubMed] [Google Scholar]
- 22. Mason SW, Barber A, Jones E, et al. . Safety and efficacy of high-dose unfractionated heparin versus high-dose enoxaparin for venous thromboembolism prevention in morbidly obese hospitalized patients [published online ahead of print December 18, 2019]. Am J Med. doi: 10.1016/j.amjmed.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 23. Wang TF, Milligan PE, Wong CA, et al. . Efficacy and safety of high-dose thromboprophylaxis in morbidly obese inpatients. Thromb Haemost. 2014;111(1):88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rondina MT, Wheeler M, Rodgers GM, et al. . Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-ill patients. Thromb Res. 2010;125(3):220-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arixtra [package insert]. Research Triangle Park, NC: GlaxoSmithKline; August 2009. [Google Scholar]
- 26. Ludwig KP, Simons HJ, Mone M, et al. . Implementation of an enoxaparin protocol for venous thromboembolism prophylaxis in obese surgical intensive care unit patients. Ann Pharmacother. 2011;45(11):1356-1362. [DOI] [PubMed] [Google Scholar]
- 27. Garcia DA, Baglin TP, Weitz JI, et al. . Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. CHEST. 2012;141(2 suppl):e24S-e43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Linkins LA, Dans AL, Moores LK, et al. . Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e495S-e530S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kearon C, Akl EA, Ornelas J, et al. . Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. [DOI] [PubMed] [Google Scholar]