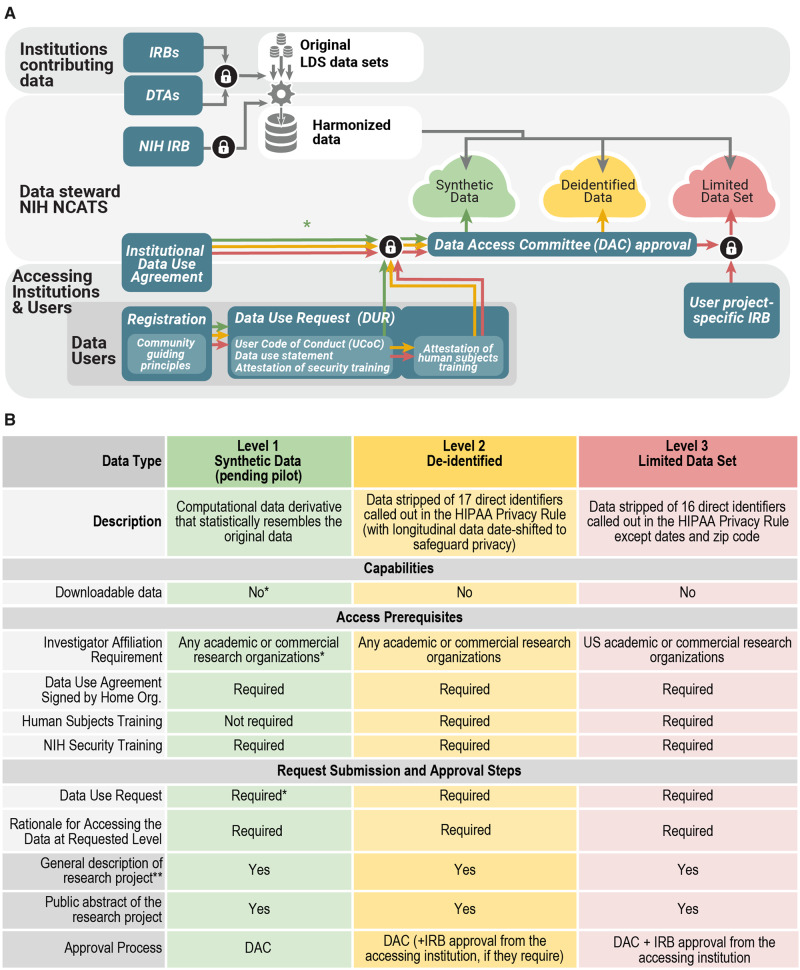
Figure 2.
Panel A. Regulatory steps and user access. Organizations can operate as data contributors or data users or both; contribution is not required for use. For contributing organizations, the first step is a Data Transfer Agreement (DTA) which is executed between National Center for Advancing Translational Sciences (NCATS) and the contributing organization (and its affiliates where applicable). For organizations using data, a separate, umbrella/institute-wide Data Use Agreement (DUA) is executed between organizations and NCATS. Interested investigators submit a Data Use Request (DUR) for each project proposal, which is reviewed by a Data Access Committee (DAC). The DUR includes a brief description of how the data will be used, a signed User Code of Conduct (UCoC) that articulates fundamental actions and prohibitions on data user activities, and if requesting access to patient-level data a proof of additional institutional review board (IRB) approval. The DAC reviews the DUR and upon approval, grants access to the appropriate data level within the National COVID Cohort Collaborative (N3C) Enclave. Synthetic data currently follow the same procedure, but if the pilot is successful, we aim to make access available by simple registration if provisioned by the organizations. The lock symbol references steps where multiple conditions must be met. HIPAA: Health Insurance Portability and Accountability Act; LDS: Limited Data Set; NIH: National Institutes of Health. Panel B. Features and requirements for each level of data in the N3C Enclave: Synthetic,35,36 De-identified data 33,34,37, and Limited Data Set, 34.