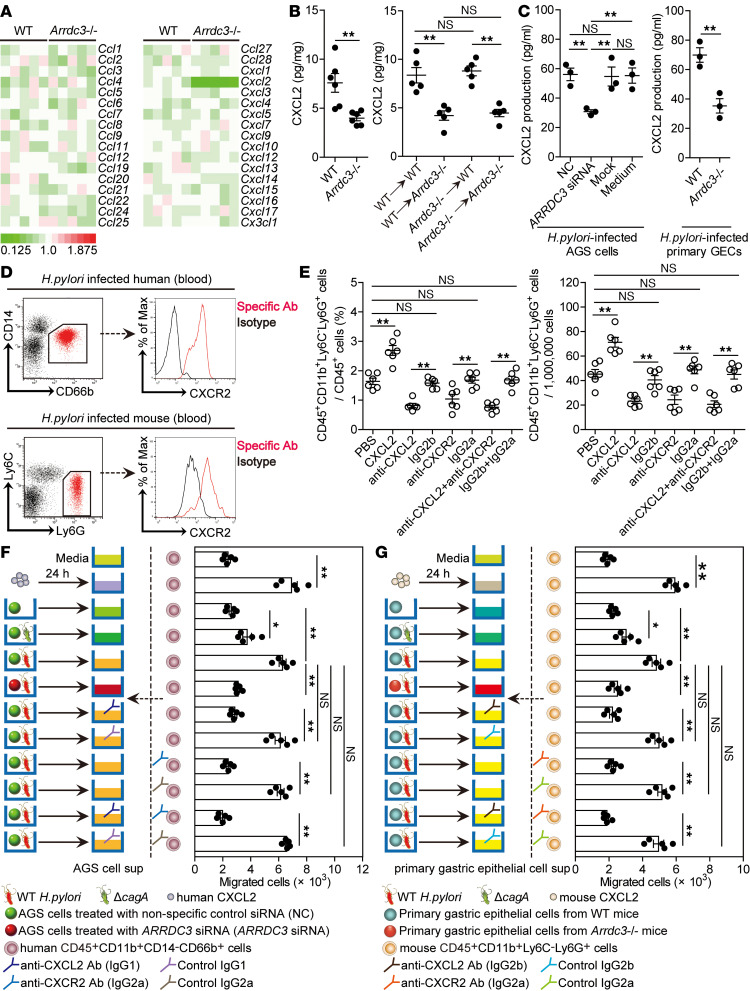
Figure 5. ARRDC3 promotes neutrophil accumulation in gastric mucosa in vivo and migration in vitro during H. pylori infection via CXCL2.
(A) The expression of chemokine family members in gastric mucosa of WT H. pylori–infected WT and Arrdc3–/– mice on day 28 p.i. was analyzed by real-time PCR (n = 5). (B) CXCL2 expression in gastric mucosa of WT H. pylori–infected WT and Arrdc3–/– mice (n = 6) or in gastric mucosa of WT H. pylori–infected BM chimera mice (n = 5) on day 28 p.i. was compared. (C) ARRDC3 siRNA, nonspecific control siRNA (NC), or lipo3000 only (mock) pretreated AGS cells or AGS cells without treatment (medium) and primary gastric epithelial cells (GECs) from uninfected WT and Arrdc3–/– mice were infected with WT H. pylori (MOI = 100) for 24 hours. CXCL2 production was measured in cell culture supernatants by ELISA (n = 3). (D) CXCR2 expression on human CD45+CD11b+CD14–CD66b+ neutrophils in blood of H. pylori–infected patients or on mouse CD45+CD11b+Ly6C–Ly6G+ neutrophils in blood of WT H. pylori–infected mice on day 28 p.i. (E) CD45+CD11b+Ly6C–Ly6G+ neutrophil levels in gastric mucosa of WT H. pylori–infected mice injected with CXCL2 or PBS control, or Abs against CXCL2 and/or Abs against CXCR2 or corresponding isotype control Abs on day 28 p.i. were compared (n = 5). (F and G) Human CD45+CD11b+CD14–CD66b+ neutrophil migration (F) and mouse CD45+CD11b+Ly6C–Ly6G+ neutrophil migration (G) was assessed by transwell assays as described in Methods and statistically analyzed (n = 5). Data are representative of 2 independent experiments. Data are mean ± SEM and analyzed by Student t test, Mann-Whitney U test, and 1-way ANOVA. *P < 0.05, **P < 0.01 for groups connected by horizontal lines. sup, supernatant.