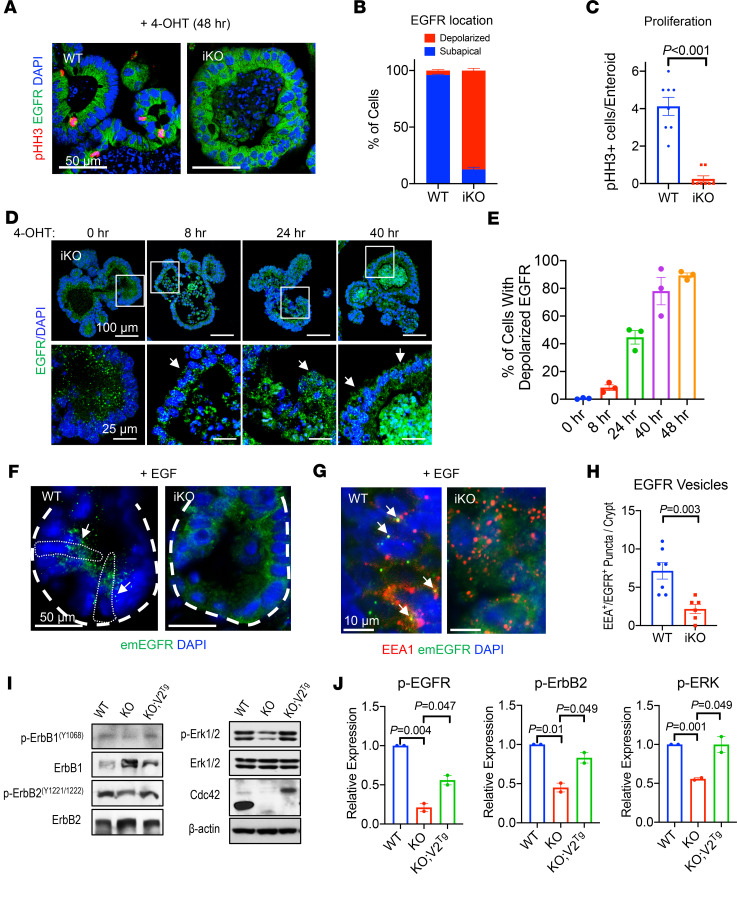
Figure 2. Cdc42 is indispensable for EGFR endocytosis.
(A) WT and Cdc42iKO enteroids were treated with 4-OHT for 48 hours. Enteroid sections were stained for endogenous EGFR (green) and mitosis marker (pHH3, red). (B) Quantification of intracellular localization of EGFR in WT and Cdc42iKO enteroid cells, based on immunofluorescence staining of EGFR in 4-OHT–treated enteroids (48 hours). (C) pHH3+ cell number was quantified per enteroid. Data in B and C were quantified from 2 independent experiments. (D) Enteroid sections were stained for EGFR after 4-OHT treatment at 8, 24, and 40 hours. White arrows point to delocalized EGFR compared with EGFR localization in untreated enteroids. (E) Quantification of percentage of cells showing changed EGFR localization at indicated time points after 4-OHT addition. (F) Cdc42 mice were crossed to emEGFR mice to visualize EGFR. Thirty minutes after EGF injection, emEGFR vesicles (green, white arrows) were seen in Cdc42-WT mouse crypt cells but were barely detected in iKO crypt cells. White dotted circles indicate crypt base stem cells. (G and H) Double fluorescence analysis for EEA1 (red) and emEGFR (green, white arrows) detected endocytic EGFR in WT crypt cells but not Cdc42iKO cells 30 minutes after EGF injection. EEA1+EGFR+ puncta per crypt were quantified from 4 mice in 2 independent experiments. (I and J) Western blots for endogenous p-ErbB1 (Y1068), p-ErbB2 (Y1221/1222), and p-Erk1/2 were performed for WT, Cdc42-KO, and Cdc42 V2Tg mouse intestines. Data represent 2 independent experiments. Please also see Supplemental Figure 2.