Fig. 2. Schematic representation of the eight possible combinations of oxygen environments in the A2+B3+2O−42 spinel structure with their electrostatic valence bond strengths calculated from Pauling’s second rule.
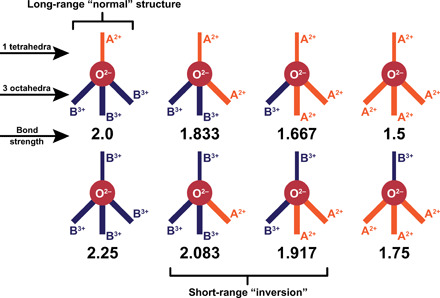
In this representation, the thinner solid line above the oxygen anion (symbolized as solid red circle) represents the single bond with the tetrahedral site, and the three thicker solid lines below oxygen represent the bonds with the octahedral sites. The orange and dark blue bonds are with A2+ and B3+ cations, respectively. Only one of the eight possible arrangements produce an electrostatic valence bond strength equal and opposed to that of the oxygen anion satisfying Pauling’s valence rule (the normal spinel arrangement, top left).
