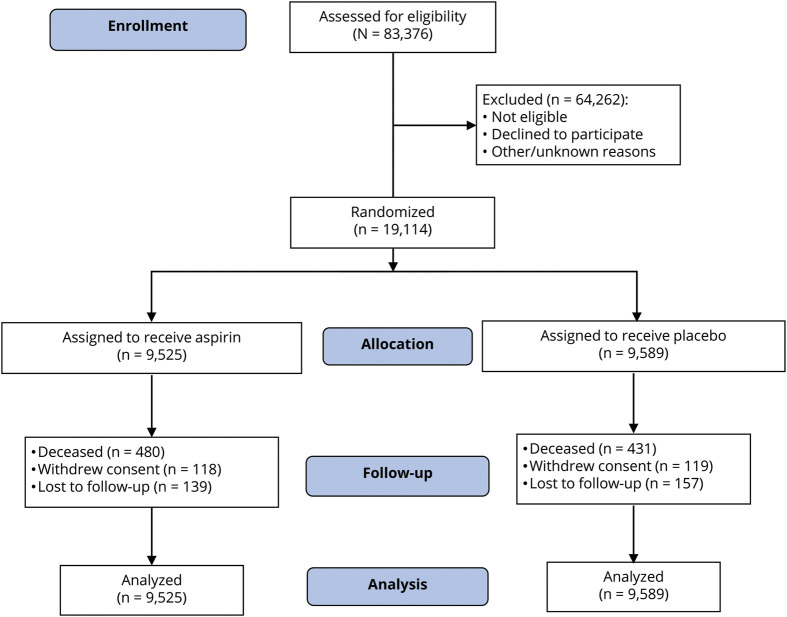
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) flow diagram of participants in the Aspirin in Reducing Events in the Elderly (ASPREE) trial.
All randomized participants were included in the final analysis. For participants who withdrew from the trial or died, all information up to the point of withdrawal/death was included in the analysis.