Abstract
Background: Pain is one of the most-frightening complications of cancer and disrupts quality of life. Cancer-related pain can be caused by primary cancer itself, metastases that occur, and interventions to treat cancer. Almost all cancer-related pain is pain with moderate-to-severe intensity. Thus, cancer-related pain management often involves administration of opioid analgesics. However, administration of opioid analgesics can cause side-effects that cause new problems for these patients. Several studies have shown that acupuncture can reduce cancer-related pain and data show that acupuncture therapy is safe and can provide clinically meaningful improvements when used in conjunction with standard therapy.
Case: A 72-year-old female patient had pain throughout her body since 1 month prior to before being admitted to the hospital. She was unable to sleep at night often cried because she was unable to stand the pain. This was reduced by morphine 3 × /day. Because of the drug's side-effects, she slept more often during the day, could not sleep at night, and was constipated. She also had breakthrough pain, on an average of 2–3 × /day. She had a history of malignancy in the pleura, liver, lungs, and cervix. There was an increase in some tumor markers. Her baseline numeric rating scale (NRS) assessment was 4 with an oral morphine slow-release tablet 3 × 15 mg/day. Acupuncture therapy was performed at Battlefield Acupuncture points of the right ear and body acupuncture points (LI 4, LI 11, ST 36, SP 6, and LR 3) was treated with 3-Hz continuous-wave electroacupuncture for 30 minutes at each session. During this therapy, there were reductions in pain (baseline NRS 4 became 2), need for morphine, morphine side-effects, and frequency of breakthrough pain. There were no significant side-effects due to acupuncture.
Conclusions: Acupuncture is an effective and safe therapeutic option for reducing cancer pain with minimal side-effects. Acupuncture can enable a reduced need for narcotic analgesics.
Keywords: acupuncture, cancer pain, battlefield acupuncture, electroacupuncture
Introduction
Pain is one of the most frightening complications of cancer and disrupts the functioning of life. Factors—including fear, anxiety, and depression—can all contribute to increased pain and breakthrough pain episodes, which result in additional medical visits and costs. The prevalence of pain in cancer reaches 50% in patients undergoing active treatment for malignancy, and up to 90% in patients with advanced cancers.1
Cancer-related pain can be caused by primary cancer itself, metastases, interventions to treat cancer (surgery, chemotherapy, and radiotherapy). However, the main cause of the pain cannot always be identified. Cancer-related pain is a mosaic of various kinds of pain that are produced through different mechanisms. The prevalence of pain is also related to the type of cancer, and age, with younger patients experiencing more pain.1,2
Almost all cancer-related pain is pain with moderate-to-severe intensity. Thus, cancer-related pain management often involves administration of opioid analgesics. However, these opioid analgesics can cause several problems, including side-effects associated with opioids, excessive doses that increase abuse/addictions, accidental overdoses, and/or respiratory depression.3
Nonpharmacologic interventions such as acupuncture are safer approaches to treating cancer-related pain. Some randomized controlled clinical trials showed that acupuncture can reduce cancer-related pain. Acupuncture therapy combined with standard therapy significantly reduces cancer-related pain, compared to standard therapy. Overall, the data show that acupuncture therapy is safe and can provide clinically meaningful improvements when used in conjunction with standard therapy.3
The International Association for the Study of Pain (IASP) classified pain into nociceptive pain and neuropathic pain. In cancer-related pain, nociceptive pain can occur as a result of invasive stimuli from the primary tumor and/or from metastatic lesions that invade or compress surrounding structures, pathologic fractures, or bleeding in tumors. Neuropathic pain is the result of changes in pain processing and occurs at the level of the central or peripheral nervous system. Examples are peripheral neuropathy induced by chemotherapy or postradiation pain. Pharmacologic management must be tailored to the specific type of pain. Often patients with cancer have mixed kinds of pain that require combination therapy.1
In nociceptive pain, after the nociceptors are stimulated, impulses are transmitted first by the nerve fibers (Aδ, thin myelinated) and then by the slower nerve fibers (C, unmyelinated). These fibers terminate in the dorsal root of the spinal cord or trigeminal ganglion, which then interact with neurons in the central nervous system (CNS) in the spinal cord. These cells synapse into the contralateral thalamus, and then, impulses are transmitted to the cortical region via the somatosensory pathway. Interactions at the cortical level are very complex, involving the somatosensory cortex, frontal cortex, and limbic system.
Any pain-impulse transmission pathway can be inhibited to block pain signals in the area. Pain perception can vary depending on several factors, including anxiety, depression, and distraction, which have no direct relationship with nociceptors or noxious stimulus, suggesting other mechanisms that modulate transduction and pain response. These mechanisms include inhibition at the level of the spinal cord by nonnoxious input (the gate-control theory) and descending inhibition of the midbrain and higher regions that contain large amounts of opioid receptors. Visceral pain arising from nociceptors in the viscera is mostly transmitted by C nerve fibers. Often less well-localized and less sharp than somatic pain, visceral pain is triggered by direct irritation from the tumor, organ distension or contraction, ischemia, necrosis, or inflammatory mediators.4
Neuropathic pain arises from injury to the nerve tissue in either the central or peripheral nervous system. This pain differs from nociceptive pain in several important ways. First, pain can occur without a preceding noxious stimulus; thus, the problem does is not associated with the nociceptors. Second, pain stimulation can occur at any place along the nerve pathway. Examples are peripheral nerves, the spinal cord, or even the central nerve. Third, chronic neuropathic pain might not have protective goals. This kind of pain is less likely to respond to nonsteroidal anti-inflammatory drug (NSAID)–based therapy or standard opioids.
In neuropathic pain—also known as a “wind-up” phenomenon—repeated stimulation of C nerve fibers causes biochemical and physical genetic changes in the CNS. As a result, damaged nerves and undamaged parts of the body can both induce pain signals through crosstalk mediated by gap junctions, evoking pain stimuli. Unlike somatic or visceral pain, the quality of neuropathic pain is often described as burning, numbness, or tingling and can be diagnosed further as allodynia or hyperalgesia. The difference between nociceptive pain (somatic and visceral) and neuropathic pain is clinically important because different therapeutic approaches are needed to achieve a good effect. Cancer-related pain can be categorized based on the mechanism of nerve damage or kind of sensation, but most cancer-related pain is mixed-type pain.4
Breakthrough Cancer Pain
Breakthrough cancer pain (BTcP) was first defined by Portenoy and Hagen in 1990 as “temporary exacerbations experienced by patients who have relatively stable and adequately controlled initial pain relief” in patients undergoing long-term opioid treatment for cancer-related pain.5,6
The current consensus defines BTcP as episodes of pain with severe intensity that occur in patients who receive routine opioid regimens for persistent pain that are sufficient to provide at least ongoing mild analgesia. The following criteria are used to diagnose BTcP: First, the patient must receive a routine opioid regimen and the initial pain must be controlled for several periods. Second, pain-trigger events are not needed to cause BTcP, but instead, should be used to describe BTcP subtypes as spontaneous or incident types.7
Spontaneous or idiopathic BTcP has no identifiable cause or precipitating event. The onset of this pain and its duration is often relatively long. Instead, Incident type of BTcP is triggered by identifiable events. This might or might not be predictable, and the pain is often relatively quick in onset and brief in duration. The most-common kinds of incident-type BTcP are bone pain due to metastatic disease and oropharyngeal pain occurring secondary to chemotherapy- or radiation-induced mucositis.7
In general, three to four episodes of BTcP per day are still considered acceptable if the pain during the rest of the day is controlled. The average number of episodes is 2.4 per day with an average pain intensity of 7.4/10. BTcP onset is more often fast (10 minutes; about 69%) than gradual (> 10 minutes). In patients who report BTcP with rapid onset, this can be predicted in about half of the cases, whereas BTcP with a gradual onset (> 10 minutes) is less predictable. Surveys repeatedly showed that most BTcP episodes peak in intensity within a few minutes and persist for 30–60 minutes.8
Biomedical Classifying and Treating Cancer-Related Pain
Initial and ongoing assessment of cancer-related pain must be an integral part of the management of patients who have cancer and should indicate when additional comprehensive assessments are needed. Regular self-reporting of pain intensity with the help of a validated assessment tool is the first step toward effective and individualized treatment. The most-commonly used standard measurement tools are the visual analogue scale (VAS), the verbal rating scale (VRS), and the numerical ranking scale (NRS).9
The Edmonton classification system for cancer pain (ECS-CP) was developed to provide a staging system for cancer pain and to estimate prognosis. In this staging system, pain is classified according to the mechanism (nociceptive or neuropathic), whether there is a breakthrough pain or not; and if the patient has psychologic stress, addictive behavior, or impaired cognitive function. Patients are then classified as levels 1–3, with level 3 having more risk factors and a less-favorable response to therapy and less-favorable prognosis. A simplified version of this classification system includes the presence or absence of the risk factors mentioned above, the presence of more risk factors (presence of nociceptive pain, neuropathic pain, or presence of breakthrough pain; as well as psychologic distress, addictive behavior, or cognitive dysfunction) indicating a poorer prognosis.1
To evaluate neuropathic pain, a douleur neuropathique (DN4) questionnaire can be used, which consists of a series of yes/no questions given by doctors regarding the characteristics of pain such as burning, tingling, numbness, or temperature sensations that change, as well as objective findings about clinical examination, such as decreased sensation of needle pricking and light touch and allodynia. Assessments are from 0 to 10, with scores >4 likely to indicate neuropathic pain.1
Cancer-pain management consists of pharmacologic and nonpharmacologic approaches. Pharmacologic treatment for cancer pain that is widely used today refers to the guidelines of the World Health Organisation (WHO).10 The WHO created a 3-step analgesic ladder in 1986 for the treatment of cancer pain to establish guidelines10 for adequate treatment of pain related to cancer, which was generally considered to have no standard of care at that time. There were concerns at the time, as exist now, about opioid addiction, and these guidelines helped greatly improve cancer-pain management worldwide. Treatment is based on the classification of mild, moderate, and severe pain, with mild pain treated with nonopioid drugs such as paracetamol, NSAIDs, or over-the-counter formulation drugs. For moderate pain, low-dose opioids may be used, and, for severe pain, high-dose opioids are recommended.
Pharmacologic therapy must also be combined with nonpharmacologic therapies such as neuropsychologic treatment, acupuncture, surgery, and integrative therapy as needed. This WHO guideline was updated in 1996 to include recommendations that first-line care is given orally when possible and that treatments are tailored to individuals.4
Common side-effects of opioids include sedation, dizziness, nausea, vomiting, constipation, physical dependence, tolerance, and respiratory depression. Dependency and physical addiction can complicate management and make it more difficult to treat advanced-cancer pain safely and adequately. Less-common side effects include delayed gastric emptying, hyperalgesia, immunologic and hormonal dysfunctions, muscle stiffness, and myoclonus. The most-common side-effects of opioid use are constipation (which has a very high incidence) and nausea. These two side-effects can be difficult to manage and, sometimes, require stopping the opioid administration; this halt contributes to underdosing and inadequate analgesia.11
The Role of Acupuncture
Acupuncture is a therapeutic modality performed by inserting fine needles at acupuncture points; medical acupuncture is an adaptation of classical Chinese acupuncture that uses the latest knowledge in the fields of anatomy, physiology and pathology, and principles of evidence-based medicine. The mechanism of action of medical acupuncture is mainly by stimulating the nervous system and includes local antidromic-axon reflexes, segmental and extrasegmental neuromodulation, and other CNS effects.12
Several meta-analysis studies suggest that acupuncture therapy combined with standard biomedical therapy reduces cancer-pain symptoms, compared to standard biomedical therapy alone.13–15 In addition, several meta-analyses of the benefits of ear acupuncture for pain relief also stated that ear acupuncture was significantly able to improve pain scores and reduce symptoms quickly.16,17
Case
A 72-year-old female patient had a chief complaint of pain throughout her body for 1 month prior to her admission to the hospital. This pain caused the patient to be unable to sleep at night; she often cried due to an inability to withstand it. Her was reduced by administration of oral morphine 3 × /day. After taking morphine for several days, the patient tended to sleep more often during the daytime, could not sleep at night, and became constipated. She had breakthrough pain an average of 2–3 times × /day. This patient also had a history of recurrent pleural effusion due to malignancy in the pleura, there were nodules in her lungs and liver suspected of being metastases, a mass in the cervix with a suspected malignancy, and breast cancer 20 years ago post radical mastectomy and chemoradiation. The malignancy in the pleura, lung, liver, and cervix is an active cancer and was only recently detected. It has not been proven whether the malignancy in the pleura, lungs, and liver is associated with active tumors in the cervix; these lesions appear to be associated with a separate, but unknown, primary tumor (i.e., a carcinoma of an unknown primary).
During her treatment the patient did not receive chemotherapy, radiation, or surgery. A physical examination showed that she was apathetic; the breathing sound in her right lung was fainter than that in the left lung; and she had a shifting dullness in her abdomen. NRS assessment showed a value of 4 of 10 with oral morphine sulfate tablets (MST) 3 × 15 mg/day. Laboratory tests showed increased values in several tumor markers (cancer antigen—125, carcinoembryonic genic antigen, cancer antigen–15-3, and Cyfra–21-1). This patients was diagnosed with cancer pain due to malignancy in her pleura, lung, liver, and cervix.
Battlefield Acupuncture (BFA) and body acupuncture points LI 4, LI 11, ST 36, SP 6, LR 3 were only applied on one side of the body to minimize stimulation to the patient's body. The BFA technique used was slightly different, which was to puncture 5 ear acupuncture points directly (Cingulate Gyrus, Thalamus, Omega 2, Point Zero, and Shen Men) in the right ear, using 0.20 × 15–mm filiform needles with 30 minutes' retention. This was done because the patient was apathetic. Needling the acupuncture points of the body was performed, using 0.25 × 25–mm filiform needles. After needling the acupuncture points of the body, 3-Hz continuous-wave electroacupuncture (EA) stimulation was performed for 30 minutes using a Hwato SDZ-V electrostimulator. Electrode wires were connected at LI 4, LI 11, ST 36, and SP 6 on one side of her body.
Results
By the third therapy session, this patient experienced reduction of her pain symptoms. She had decreases in her NRS score (to 2) and had less need for morphine—on some days, she was not even given morphine. Thus, she had reduced morphine side-effects (she did not sleep during the day and was able defecate after a few days of no morphine). She received 8 acupuncture treatments and experienced a decrease in the frequency of her BTcPs to 1 × /day. There were no significant side-effects of this acupuncture therapy.
Discussion
Battlefield Acupuncture (BFA) was developed by Richard C. Niemtzow in 2001 research to find a more efficient auriculotherapy system for pain relief that works very fast.18 This technique results in a very significant reduction in pain within minutes. The duration of the pain-free period varies from minutes to hours, days, weeks, and months depending on the pathology that occurs and the duration of stimulation; the needle, EA, and laser excitation of the ear acupuncture points also influence the duration of the pain-free time. Ear acupuncture points are stimulated sequentially as follows: Cingulate Gyrus; Thalamus; Omega 2; Point Zero; and Shen Men. Most likely, the BFA method affects the processing and modulation of pain in the CNS involving the hypothalamus, thalamus, cingulate gyrus, and cerebral-cortex structures. Functional magnetic resonance imaging (fMRI) research conducted by Cho showed the involvement of these structures.18
Usichenko et al. conducted a systematic review, in 2017, of 17 RCTs to determine which ear acupuncture points were most-used in pain management. The researchers found that 15 of the 20 ear acupuncture points most widely used for pain management are located in the area of the external auricula which is innervated by the auricular branch of the vagal nerve (ABVN), while the points used as a comparison sham are mostly located in the area helices and lobules, which are innervated by the cervical nerve. ABVN stimulation in the external auricula produces a cardiovascular response that can be blocked by administering acetylcholine receptor muscarinic antagonists such as atropine. In addition, the fMRI examination showed activation of the solitary nucleus and locus coeruleus and decreased activity of the amygdala, hippocampus, parahippocampal gyrus, and cingulate cortex. This suggests that ear acupuncture likely stimulates the ABVN and affects the body's organs through the autonomic system, while the decrease in pain caused by ear acupuncture might arise from regulation of the autonomic system and regulation of the emotional component of the pain.19 Figure 1 shows the BFA points used, and Figures 2–6 show the body acupuncture points used.
FIG. 1.
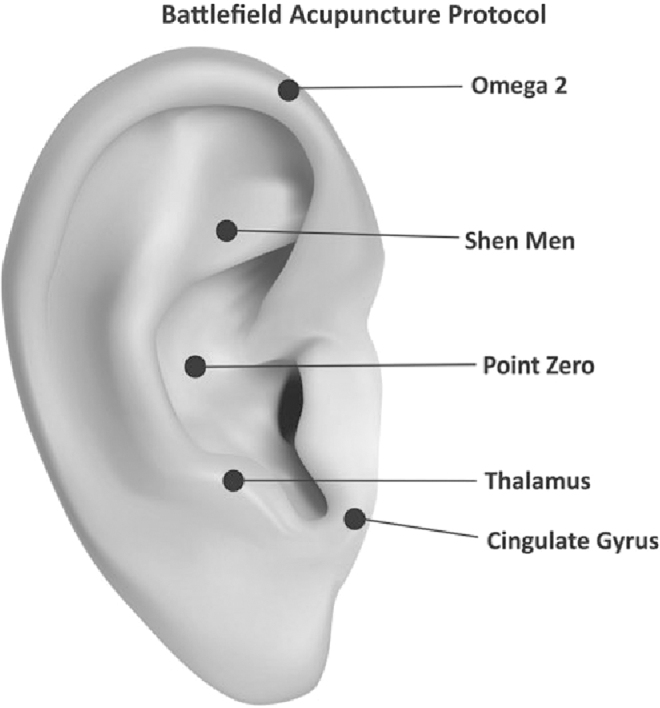
Battlefield Acupuncture points.
FIG. 2.
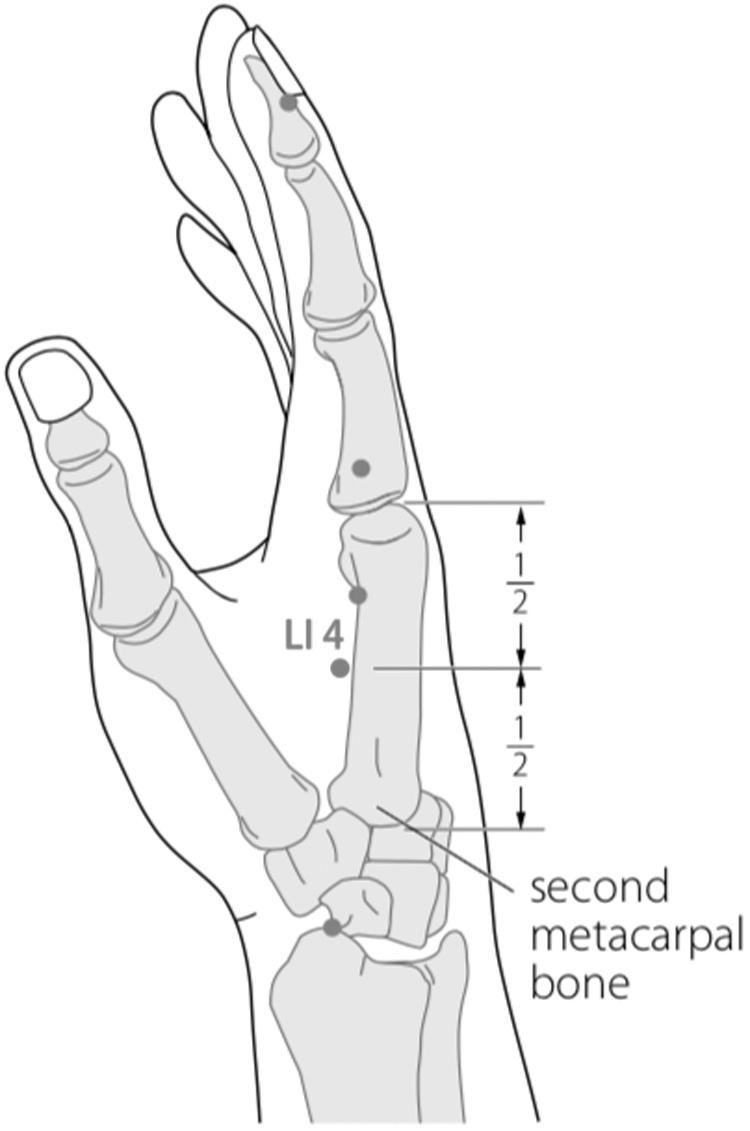
Body acupuncture: LI 4 Hegu.
FIG. 6.
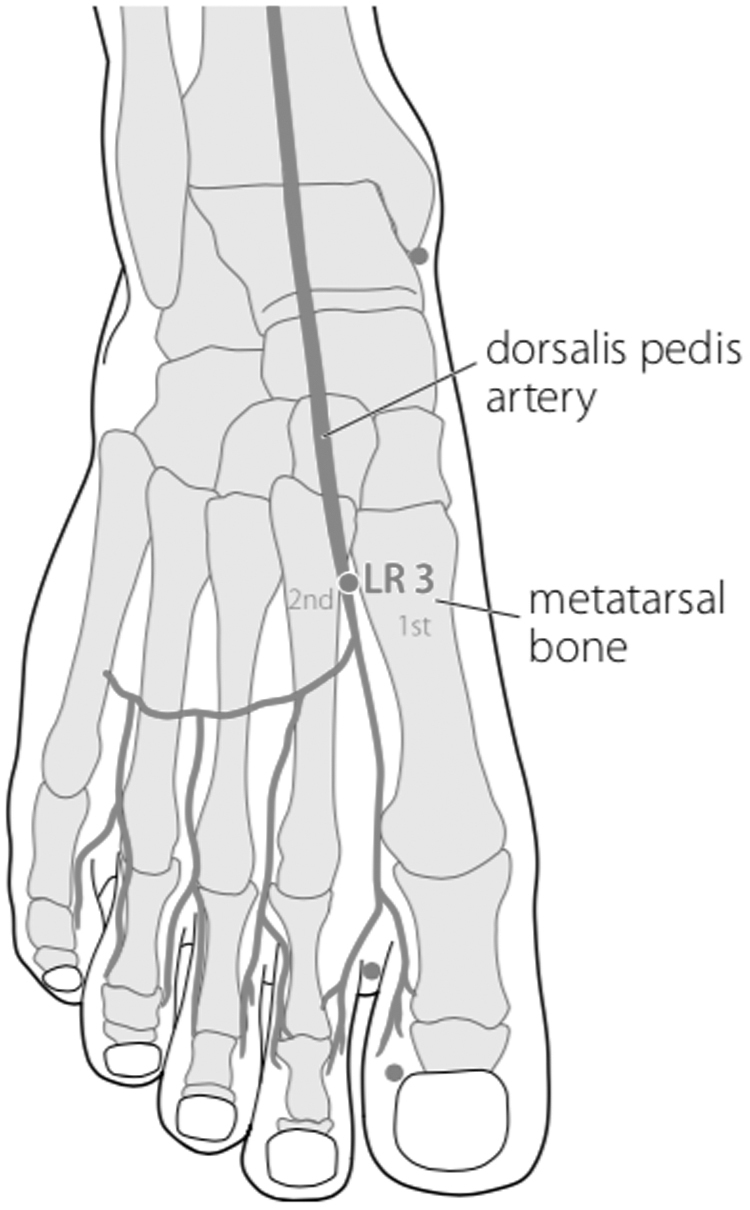
Body acupuncture: LR 3 Taichong.
FIG. 3.
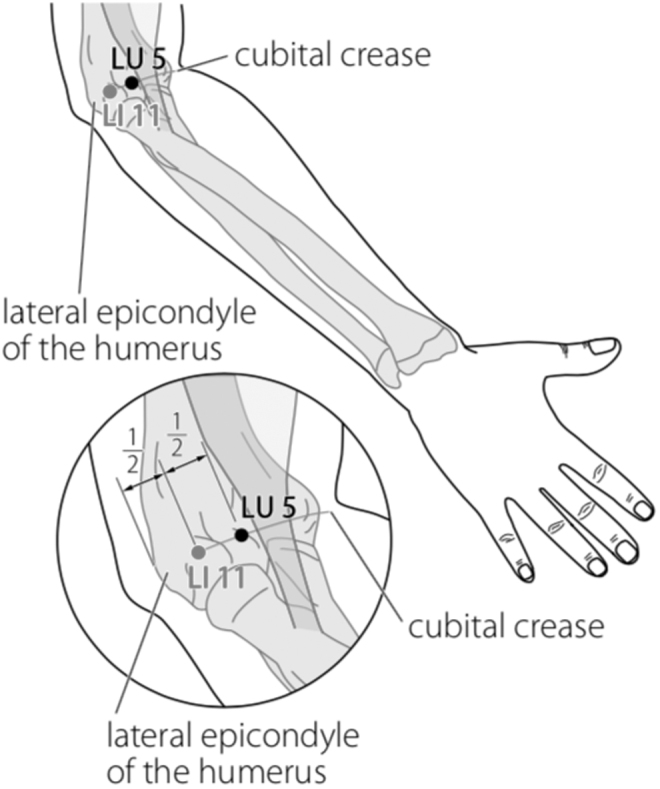
Body acupuncture: LI 11 Quchi.
FIG. 4.
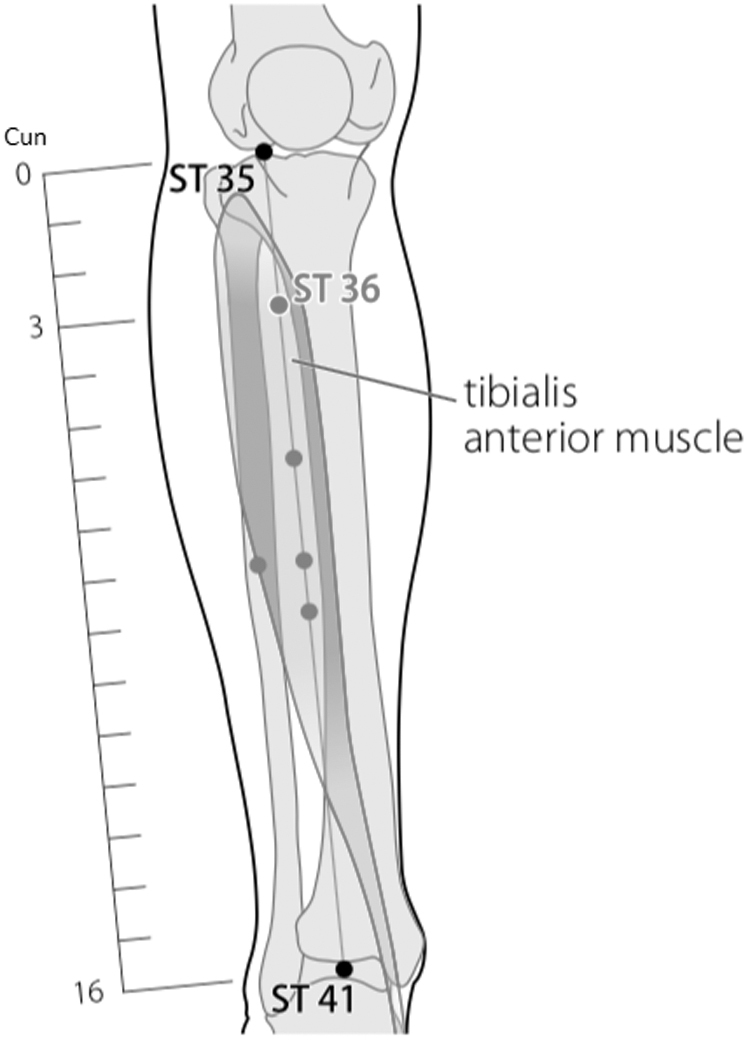
Body acupuncture: ST 36 Zusanli.
FIG. 5.
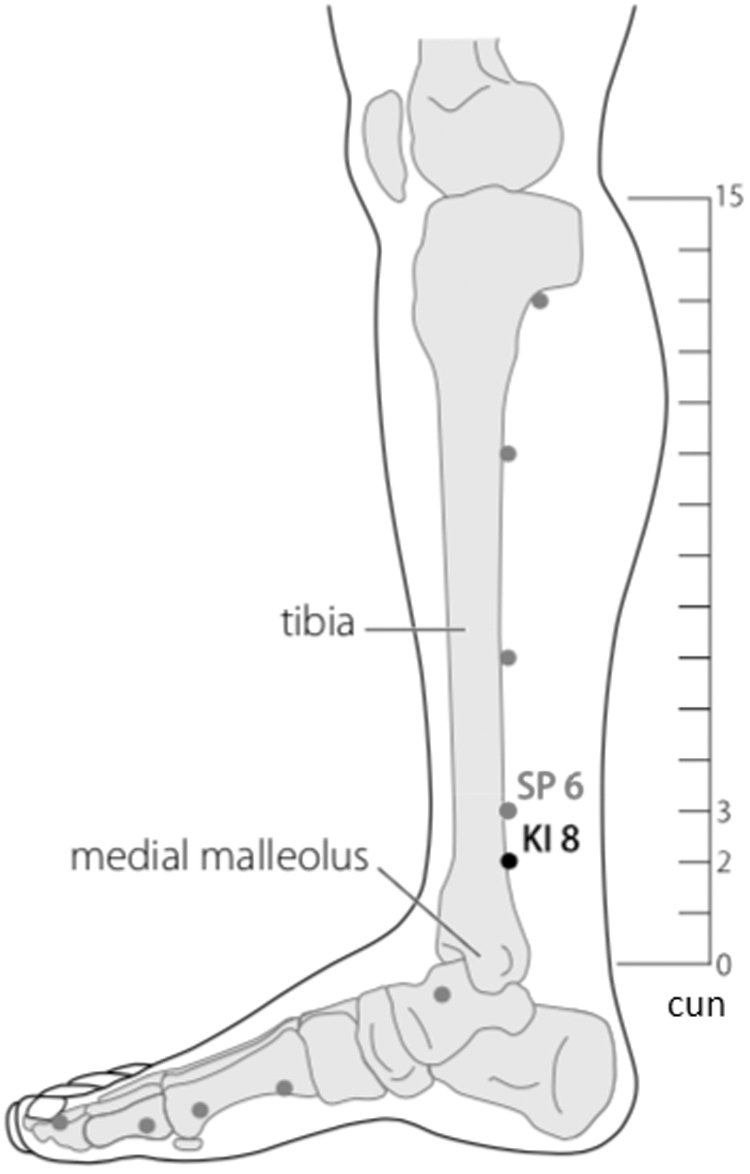
Body acupuncture: SP 6 Sanyinjiao.
Stimulated acupuncture points for the current patient were LI 4, LI 11, ST 36, SP 6, and LR 3, which have evidence-based analgesic effects. In this patient, point selection was only on one side of the body to minimize the stimulation.
The reason for choosing LI 4 was that it activates the hypothalamus and pituitary to secrete endogenous opioid peptides (β-endorphins, enkephalins, and dynorphins) that play a role in analgesia, reducing levels of substance P, calcitonin gene-related peptide (cGRP), natural killer–1 mRNA, cyclo-oxygenase-1 mRNA, and prostaglandin-E2 in the spinal cord. In the fMRI studies that examined the needling effects of LI 4, there was some activation of various brain areas, such as the somatosensory cortex (superior parietal lobe and postcentral gyrus), limbic system (calcarina gyrus, precuneus, cingular cortex, and parahippocampal gyrus), visual cortex (superior occipital gyrus), pain area (calcarina gyrus, precuneus, cingula cortex, and parahippocampal gyrus), and cerebellum which play roles in processing pain and emotions.20
ST 36 was chosen because it can reduce the activity of TRPV1 in the dorsal root ganglion to reduce pain. EA at ST 36 can inhibit an impulse-induced noxious stimulus from a single fiber from the dorsal root mediated by sympathetic nerves. In addition, stimulation of EA at ST 36 can produce inhibitory postsynaptic potentials and long-standing membrane hyperpolarization of nociceptive neurons in the dorsal horn of the spinal cord, which has caused inhibition of nociceptive response to noxious thermal stimuli in cat feet.21 This suggests the possible involvement of postsynaptic inhibition in the analgesic effect of EA at point ST 36.21
The combination of ST 36 and SP 6 can reduce the hypersensitivity condition in neuropathic pain through the involvement of the opioid system, and the effectiveness of analgesia is equivalent to gabapentin.22 And LR 3 together with LI 4 has proven to be effective for reducing pain.
In general, acupuncture reduces pain through three mechanisms, namely, local, segmental, and central effects. Piquiring the needle at an acupuncture point will cause microtrauma, which will trigger the body to release various mediators, including substance P, β-endorphins, and cGRP. Mast cells through nitric-oxide regulation secrete serotonin, cytokines, and histamine.
At the segmental level, the stimulation will stimulate the Aδ nerve in turn, a stimulation that will then be delivered to marginal cells in the spinal cord and then transmitted to stalk cells through serotoninergic fibers (5-hydroxytryptophan). Stalk cells will inhibit the substantia gelatinosa through an enkephalinergic mechanism so that pain impulses carried by afferent C-nerve fibers will be inhibited and this will be transmitted to a wide dynamic range that delivers stimulation through axons to the reticular formation through the spinoreticular tract. The impulses from marginal cells will be transmitted to the ventroposterior thalamus nucleus, which will then be projected to the cerebral cortex. In the brainstem, there is a collateral branch to the periaqueductal grey matter that projects the stimulation to a lower level, namely, the nucleus raphe magnus and the paragigantocellular nucleus in the medulla oblongata, which will then stimulate serotonergic and noradrenergic fibers to the stalk cells so that they will effect transmission inhibition on the substantia gelatinosa, giving rise to an anti-nociceptive effect.12
The current patient was treated with low-frequency continuous-wave EA because it could stimulate the release of β-endorphins, which have analgesic effects. EA at 2 Hz is very effective for accelerating release of β-endorphin and encephalin, with high selectivity for μ- and δ-receptors in the CNS. EA 2 at Hz is also effective for accelerating release of brain endomorphins, which induce antinociceptive effects. In mice with neuropathic pain, EA at 2 Hz induced a strong and long-lasting analgesic effect, compared to 100 Hz. Also, μ- and δ- receptors, but not κ-receptors, mediated the analgesic effect caused by EA at 2 Hz.21
Several recent studies have shown that both manual acupuncture (MA) and EA can affect leukocytes and proinflammatory cytokines. However, laboratory examinations revealed that EA was more effective than MA for reducing proinflammatory cytokines interleukin-6, interferon-γ, and tumor necrosis factor–α in subjects with arthritis and collagen-induced inflammation. The analgesic mechanism of EA is related to the frequency of electrical stimulation. It is known that EA with a lower frequency (2 Hz) induces the release of enkephalin and β-endorphin, whereas EA with a higher frequency (100 Hz) induces the release of dynorphine. Recent research showed that different EA frequencies had different effects on normal people and patients with chronic inflammation. Lower frequencies (2–10 Hz) were more effective than higher frequencies (100 Hz) for suppressing inflammatory pain. In other words, for patients with chronic inflammatory pain and neuropathic pain, it is better to use lower frequency EA than higher frequency EA.23
Conclusions
Acupuncture is one of the nonpharmacologic treatment modalities that can be performed on patients who have cancer-related pain because acupuncture has an analgesic effect that is equivalent to the analgesic effect of morphine, but induces with minimal side-effects and that improve the quality of life of patients who have cancer.
Author Disclosure Statement
No financial conflicts of interest exist.
Funding Information
The funding of the present work was wholly provided by the first author.
References
- 1. Money S, Garber B. Management of cancer pain. Curr Emerg\ Hosp Med Rep. 2018;6(4):141–146 [Google Scholar]
- 2. Wiffen PJ, Derry S, Moore RA, et al. . Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev. 2017;7:CD012637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller KR, Patel JN, Symanowski JT, Edelen CA, Walsh D. Acupuncture for cancer pain and symptom management in a palliative medicine clinic. Am J Hosp Palliat Care. 2019;36(4):326–332 [DOI] [PubMed] [Google Scholar]
- 4. Smith TJ, Saiki CB. Cancer pain management. Mayo Clin Proc. 2015;90(10):1428–1439 [DOI] [PubMed] [Google Scholar]
- 5. Portenoy RK, Hagen NA. Breakthrough pain: Definition, prevalence and characteristics. Pain 1990;41(3):273–281 [DOI] [PubMed] [Google Scholar]
- 6. Working Group Nientemale DEI; Vellucci R, Fanelli G, Pannuti R, et al. . What to do, and what not to do, when diagnosing and treating breakthrough cancer pain (BTcP): Expert opinion. Drugs. 2016;76(3):315–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mercadante S, Portenoy RK. Breakthrough cancer pain: Twenty-five years of study. Pain. 2016;157(12):2657–2663 [DOI] [PubMed] [Google Scholar]
- 8. Mercadante S. Breakthrough pain in cancer patients: Prevalence, mechanisms and treatment options. Curr Opin Anaesthesiol. 2015;28(5):559–564 [DOI] [PubMed] [Google Scholar]
- 9. Fallon M, Giusti R, Aielli F, et al. . Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29(suppl4):iv166–iv191 [DOI] [PubMed] [Google Scholar]
- 10. Balding L. The World Health Organisation analgesic ladder: Its place in modern Irish medical practice. Ir Med J. 2013;106(4):122–124 [PubMed] [Google Scholar]
- 11. Benyamin R, Trescot AM, Datta S. Opioid complications and side effects. Pain Physician. 2008;11(2[suppl]):S105–S120 [PubMed] [Google Scholar]
- 12. White A; Editorial Board of Acupuncture in Medicine. Western medical acupuncture: A definition. Acupunct Med. 2009;27(1):33–35 [DOI] [PubMed] [Google Scholar]
- 13. Chiu HY, Hsieh YJ, Tsai PS. Systematic review and meta-analysis of acupuncture to reduce cancer-related pain. Eur J Cancer Care (Engl). 2017;26(2):12457. [DOI] [PubMed] [Google Scholar]
- 14. Lau CH, Wu X, Chung VC, et al. . Acupuncture and related therapies for symptom management in palliative cancer care: Systematic review and meta-analysis. Medicine (Baltimore). 2016;95(9):e2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu C, Zhang H, Wu W, et al. . Acupuncture for pain management in cancer: A systematic review and meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1720239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murakami M, Fox L, Dijkers MP. Ear acupuncture for immediate pain relief—a systematic review and meta-analysis of randomized controlled trials. Pain Med. 2017;18(3):551–564 [DOI] [PubMed] [Google Scholar]
- 17. Jan AL, Aldridge ES, Rogers IR, Visser EJ, Bulsara MK, Niemtzow RC. Does ear acupuncture have a role for pain relief in the emergency setting? A systematic review and meta-analysis. Med Acupunct. 2017;29(5):276–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niemtzow RC. Battlefield Acupuncture. Med Acupunct. 2007;19(4):225–228 [Google Scholar]
- 19. Usichenko T, Hacker H, Lotze M. Transcutaneous auricular vagal nerve stimulation (taVNS) might be a mechanism behind the analgesic effects of auricular acupuncture. Brain Stimul. 2017;10(6):1042–1044 [DOI] [PubMed] [Google Scholar]
- 20. Hsieh JC, Tu CH, Chen FP, et al. . Activation of the hypothalamus characterizes the acupuncture stimulation at the analgesic point in human: A positron emission tomography study. Neurosci Lett. 2001;307(2):105–108 [DOI] [PubMed] [Google Scholar]
- 21. Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–375 [DOI] [PubMed] [Google Scholar]
- 22. Cidral-Filho FJ, da Silva MD, Moré AO, Córdova MM, Werner MF, Santos AR. Manual acupuncture inhibits mechanical hypersensitivity induced by spinal nerve ligation in rats. Neuroscience. 2011;193:370–376 [DOI] [PubMed] [Google Scholar]
- 23. Jin BX, Jin LL, Jin G-Y. The anti-inflammatory effect of acupuncture and its significance in analgesia. World J Acupunct–Moxibustion. 2019;29(1):1–6 [Google Scholar]


