Fig. 3. TIF effect.
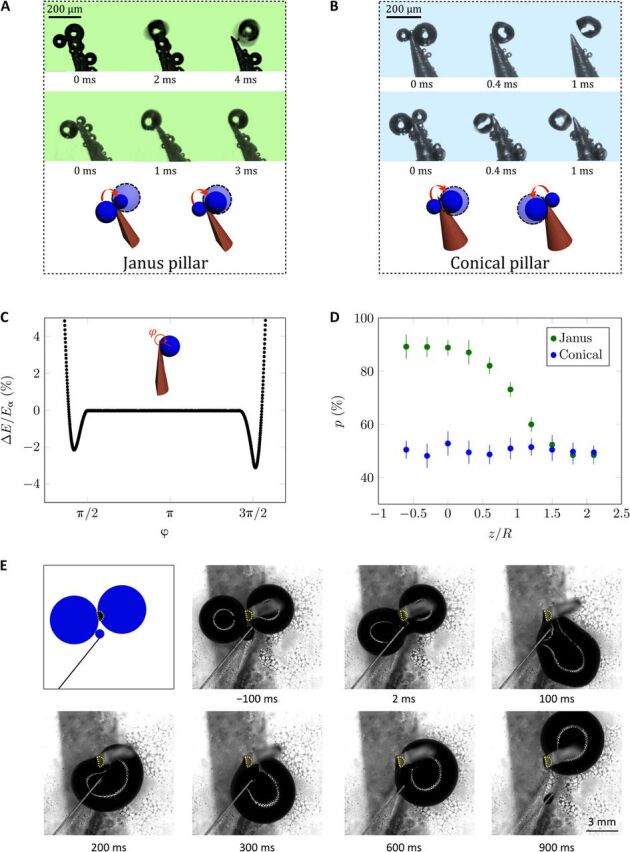
(A) TIF (TIF effect) on Janus pillars: Droplets tend to accumulate at the pillar tip (movie S5), either directly on the curved side of the pillar (first picture) or after flipping from flat to curved (second picture). In the latter case, large droplets can be attracted by small ones, showing that the TIF effect overcomes the difference of Laplace pressure between droplets (movie S6). (B) On conical pillars, droplets also gather at the pillar tips, but they sit indifferently on the right or left side of the pillar (movies S7 and S8). Photo credit: Shile Feng, City University of Hong Kong. (C) Droplet surface energy (compared to that in air) as a function of φ, the flip angle around the Janus tip. ΔE is normalized by Ea, the adhesion energy of the droplet on a flat solid. (D) Probability p to find a droplet on the curved side of a Janus pillar as a function of z/R, the normalized distance to the tip (green data). p is deduced from statistics performed on 200 droplets and compared to the probability of finding droplets on the right side of conical pillars (blue data). (E) Sketch and top view of the coalescence of two water drops with R ≈ 2.5 mm sitting on a superhydrophobic material and placed respectively on the flat and curved sides of a vertical hemicylinder (with r = 0.5 mm), whose profile is highlighted with dots. Time starts at the onset of coalescence, after which the merged drop rotates around the solid until it pins on its curved side, confirming the generality of the TIF effect (movie S9). Photo credit: Antoine Malod, ESPCI Paris.
