Abstract
The development of insects is strongly influenced by their resident microorganisms. Symbionts play key roles in insect nutrition, reproduction, and defense. Bacteria are important partners due to the wide diversity of their biochemical pathways that aid in the host development. We present evidence that the foam produced by nymphs of the spittlebug Mahanarva fimbriolata harbors a diversity of bacteria, including some that were previously reported as defensive symbionts of insects. Analysis of the microbiomes in the nymph gut and the soil close to the foam showed that the microorganisms in the foam were more closely related to those in the gut than in the soil, suggesting that the bacteria are actively introduced into the foam by the insect. Proteobacteria, Actinobacteria, and Acidobacteria were the predominant groups found in the foam. Since members of Actinobacteria have been found to protect different species of insects by producing secondary metabolites with antibiotic properties, we speculate that the froth produced by M. fimbriolata may aid in defending the nymphs against entomopathogenic microorganisms.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00211-1) contains supplementary material, which is available to authorized users.
Keywords: Actinobacteria, Bacterial diversity, Hemiptera, Insect microbiome, Symbiosis
Introduction
Spittlebugs are cercopid insects that in the nymph stage produce a distinctive foam while they feed on the host plants [1, 2]. The foam is produced by sucking air into the ventral cavity of the abdomen, which is then trapped in the fluid of the Malpighian tubules, resulting in the formation of bubbles in the terminal part of the abdomen [1, 3]. The foam comprises liquid, air, and surface-active molecules that reduce surface and interfacial tension to form emulsions [4–6].
Several functions have been attributed to the foam, such as the protection of nymphs against high temperatures [6], desiccation [7], and natural enemies [7, 8]. The spittlebug Mahanarva fimbriolata feeds on sugarcane roots exposed on the soil surface or belowground [9, 10]. Because of the humid nature of the foam and its proximity to the soil, there is a continuous threat of fungal and bacterial infestation, such as by the entomopathogenic fungus Metarhizium anisopliae, which occurs naturally in soils of sugarcane fields where M. fimbriolata develops [11]. The means by which the nymphs are protected from microbial attack during this stage are little understood. Associations between insects and beneficial microorganisms as a protective adaptation are common [12–15]. The mechanisms by which symbionts can protect their hosts from natural enemies are diverse and not mutually exclusive, and include competition for nutrients or space; activation of the insect immune system, which may be more deleterious to the invader than to the resident microbiome; and the production of secondary metabolites with antibiotic properties [14, 15]. An example of this last is the females of the European beewolf (Philantus triangulum), which cultivate Streptomyces spp. in specialized antennal glands and apply them to the brood cell prior to oviposition in sandy soil, where the bacteria protect against the attack of entomopathogenic microorganisms. Survival of the nymphs is reduced from 80 to 10% if the Streptomyces cells are removed [16].
Actinobacteria is the main group of bacteria that have been found to defend insects against microorganisms, by producing antimicrobial compounds [17]. Proteobacteria, including Gammaproteobacteria and Alphaproteobacteria, are also involved in protecting some hemipterans against natural enemies [12, 18, 19]. Therefore, one possible means for such a protective function in M. fimbriolata would be the presence of beneficial microorganisms in the foam covering the nymphs.
It has long been recognized that the gut microbiome is involved in the growth, development, and adaptation of the insect host [20–22]. The insect gut can be considered a portion of the external environment in which the conditions and resources are controlled by the insect [23]. A community of Actinobacteria has been reported in the gut of some hemipterans, such as firebugs and pentatomids [24, 25]. Spittlebugs are widely recognized as dependent on symbiosis with bacteria for nutritional reasons [26–28]. However, little is known about the microbiome in the gut of M. fimbriolata nymphs.
The overall aim of this study was to assess the diversity and composition of the bacterial community present in the foam produced by nymphs of M. fimbriolata, comparing it to communities found in their gut and in the soil close to the foam. We also discuss the likely protective role of bacteria in the foam, based on their taxonomic group.
Materials and methods
Foam, soil, and gut sampling
Foam produced by fourth- and fifth-instar nymphs of M. fimbriolata was collected in a sugarcane field (Saccharum officinarum L.), cultivar “SP80-1842”, a nontransgenic crop, in Piracicaba, Brazil (22° 42′ 06″ S, 47° 33′ 50″ W). The sugarcane plants were approximately 2-m tall, with 1 m between rows. The foam from five insects was pooled to produce each replicate. For comparison, the soil close to the foam was also collected. For the analysis of gut contents, fourth- and fifth-instar nymphs were collected and carefully transferred to the roots of sugarcane plants for transport to the laboratory and subsequent dissection. Insects were surface-sterilized using 0.5% sodium hypochlorite in 70% ethanol (v/v), followed by washes in sterile deionized water under aseptic conditions. Surface-sterilized nymphs were dissected in a laminar-flow hood in sterile 0.85% NaCl saline solution to obtain the whole guts. Pools of guts, each from five insects, were placed in tubes (1.5 ml) containing 1 ml of absolute ethanol [29]. A total of 15 samples (five from each environment, i.e., foam, gut, and soil) were used to perform the quantification of total bacterial community. The distance between the samples was approximately 10 m. For the sequencing of the 16S rRNA gene, 3 replicates of each environment were analyzed. All samples were maintained at − 20 °C prior to DNA extraction.
DNA extraction of total bacterial community
DNA from the samples of foam, gut, and soil was extracted using the Power Soil DNA Isolation kit (MoBio, Carlsbad, USA) according to the manufacturer’s instructions. For gut samples, the material was removed from the freezer, triturated with sterile pestles, and the triturated material was used in the extraction procedure. In all cases, aliquots of 0.5 g were used for DNA extraction. DNA preparations were visualized by electrophoresis in 1% agarose gel in 1× TAE (Tris-Acetate-EDTA buffer) to assess yield and integrity. The samples were then stored at − 20 °C until the following analysis.
Quantification of total bacterial community
The abundance of the total bacterial community was estimated by using the 16S rRNA partial gene as a proxy. Each sample was quantified twice, using the StepOne Real Time System (Applied Biosystems) with SYBR Green I. The reaction was performed in 25 μl of the reaction mixture from a Power SYBR Green PCR Master Mix (Applied Biosystems, Frankfurt, Germany), 0.5 μl of each primer (100 μm), and 1 μl of target DNA (≈ 10 ng). The primers used were P1/P2 [30] and the thermal cycling conditions were 35 cycles at 94 °C for 30 s of denaturation, followed by 55 °C for 30 s of annealing and 72 °C for 30 s in a final extension. The standard curve was constructed using serial dilutions (108 to 101) of a DNA of environmental soil samples where the concentration was previously determined by Qubit Fluorometric Quantification (ThermoFischer, USA). The amplification efficiency was 92.60% and r2 = 0.995. Data from the DNA amplification was interpolated on a standard curve, and the number of copies of the target gene was calculated in relation to nanograms of DNA in each sample. Specificity of the amplification products was confirmed by melting-curve analysis, and the expected sizes of the amplified fragments were checked in 1% agarose gel.
Sequencing of the 16S rRNA gene
The DNA extracted from the foam, gut, and soil samples (3 replicates of each) was used for PCR amplification, using the primers S-D-Bact-0341-b-S-17 and S-D-Bact-0785-a-A-21 [31] (coupled with Illumina adapters), which cover the hypervariable regions V3–V4 of the 16S rRNA gene. Amplification, pooling, and purification were performed at the University of São Paulo, Brazil (http://genfis40.esalq.usp.br/multi/). All samples were sequenced using a MiSeq Platform and the Nextera XT index kit for library preparation (Illumina, USA).
Next-generation sequence analysis
The sequenced paired-end reads were separated by sample and analyzed using the QIIME software and BPM pipeline [32, 33]. Quality-control analyses were performed to eliminate low-quality reads, short reads, and chimeric sequences, and to trim the low-quality 3′ region of individual reads in order to achieve a minimum quality of Q28 (Phred scale). The remaining sequences were clustered in operational taxonomic units (OTUs) at 97% sequence identity using VSEARCH 6.1 (v6.1.544), followed by selection of a representative sequence for each OTU [34]. The reads were then aligned with the Greengenes Core Set [35], using the PyNAST algorithm, and filtered. An OTU table was generated, singletons were excluded, and the OTU table was rarefied (50,000 sequences per sample) to prevent bias related to the different number of reads in the samples. The analysis was realized using the package “phyloseq.” All sequences have been deposited to the MG-RAST under accession numbers mgm4796924.3, mgm4796929.3, mgm4796930.3, mgm4796931.3, mgm4796932.3, mgm4796933.3, mgm4796934.3, mgm4796935.3, and mgm4796936.3.
Statistical analyses
Statistical analyses were performed using R [36]. Significant differences in gene abundance were identified using analysis of variance (ANOVA) followed by Tukey’s post hoc test, using the “ExpDes” package [37]. The differential abundance of bacterial groups was analyzed using the “edgeR” package [38]. A Venn diagram was constructed in order to determine the proportion of groups that were exclusive and shared between samples, using the webtool developed by Bioinformatics & Evolutionary Genomics (http://bioinformatics.psb.ugent.be/webtools/Venn/). Principal coordinates analysis (PCoA) ordination was performed based on the Bray-Curtis ecological distance. The matrix originated by the PCoA was used to calculate the permutational multivariate analysis of variance (PERMANOVA). These analyses were performed based on OTU-generated matrices exported into Microbiome Analyst [39].
Results
Abundance of total bacterial community
The abundance of the total bacterial community ranged among the sampled sites from 8.61 to 9.93 log of copies of 16S rRNA gene/g of sample (Table 1). Bacteria were most abundant in the soil samples (log values of 9.93 copies of 16S rRNA/g of soil). Bacterial abundance was similar in the foam and gut samples (log values of 8.70 and 8.61 copies of 16S rRNA, respectively), with no statistical difference according to the ANOVA + Tukey test.
Table 1.
Abundance of the total bacterial community present in the soil close to the foam in a sugarcane field, in the foam produced by Mahanarva fimbriolata nymphs, and in the gut of nymphs (n = 5 per sampled site)
| Samples | Log of copies of 16S rRNA/g of sample |
|---|---|
| Soil | 9.93 ± 0.01a |
| Foam | 8.70 ± 0.03b |
| Gut | 8.61 ± 0.02b |
Different letters correspond to statistical differences between means detected by ANOVA + Tukey test (P < 0.05)
Bacterial communities in foam, gut, and soil samples
After quality filtering, a total of 50,000 high-quality sequences per sample were obtained. A mean Good’s coverage of 99% was determined, indicating that the dataset was representative of the bacterial community analyzed (Table S1).
Evaluating the differences in microbial community structure, the relative abundance of OTUs in different samples was used to compute a Bray-Curtis dissimilarity matrix coordinated with PCoA. The contrasting patterns observed indicated that the microbiome was composed differently in the three environments sampled (Fig. 1). The main difference was between the foam samples from gut and from soil (observed on the first axis), while the difference between gut and soil samples was observed when two axes were used to plot samples (Fig. 1). The replicates of soil samples showed higher variability than the replicates from the other sites (gut and foam), indicating a stricter selection of the microbial communities composing the foam and gut microbiomes. The differences among the communities were further confirmed by PERMANOVA (F value = 3.363; r2 = 0.528; P < 0.006).
Fig. 1.

Principal coordinates analysis (PCoA) based on the differences in microbial community structures in the foam produced by Mahanarva fimbriolata nymphs, in the gut of nymphs, and in the soil close to the foam in a sugarcane field. The values on the axes indicate the percentage of the variance represented on each axis. Distinctions among communities were confirmed by PERMANOVA (F value = 3.363; r2 = 0.528; P < 0.006)
The Venn diagram showed that the majority of OTUs were found in only one environment. Twenty-eight OTUs were shared among the foam, gut, and soil samples. Forty-eight OTUs were shared between samples of foam and gut, while foam and soil samples shared 24 OTUs, and soil and gut shared only 8 OTUs. Foam samples hosted 257 specific OTUs, while gut samples hosted 291 specific OTUs and soil hosted 288 specific OTUs (Fig. 2).
Fig. 2.
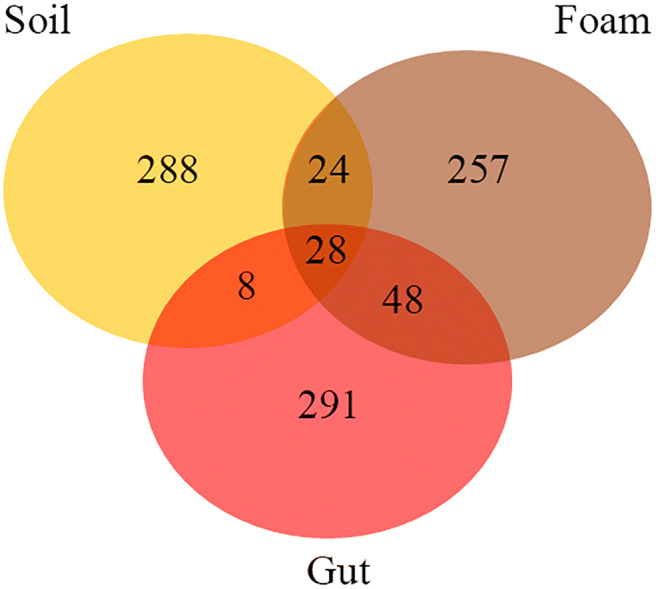
Venn diagram constructed to determine the proportion of operational taxonomic units (OTUs) of bacteria exclusive to and shared in the foam produced by Mahanarva fimbriolata nymphs, in the gut of nymphs, and in the soil close to the foam in a sugarcane field. The data were analyzed using the webtool developed by Bioinformatics & Evolutionary Genomics (http://bioinformatics.psb.ugent.be/webtools/Venn/)
Metrics of alpha-diversity
Both the foam and the gut samples were more diverse than the soil samples (Table S1). The highest mean values of the Shannon index were observed for foam samples (8.69), followed by gut (8.08) and soil samples (2.26) (P < 0.05) (Table S1). Analysis of the sample richness using the Chao1 index showed that the spittlebug-derived samples showed higher mean richness than soil samples. Samples from foam showed Chao1 values of 172,333, gut samples showed 132,993, and soil samples were estimated to harbor 698 species (Table S1) (P < 0.05).
Identification of bacterial taxa in samples
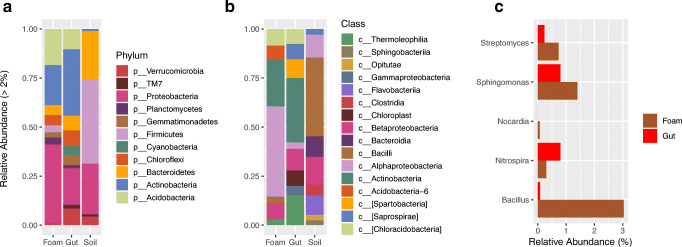
Considering the bacterial phyla represented by more than 3% of the sequences, the major bacterial groups found in soil were Proteobacteria (38.1%), Bacteroidetes (37.3%), and Firmicutes (11.1%). Samples from foam were composed mainly of Proteobacteria (40.3%), Actinobacteria (19.5%), and Acidobacteria (17.0%). In gut samples, the most prevalent phyla were Actinobacteria (33.7%), Proteobacteria (16.6%), and Acidobacteria (10.1%) (Fig. 3a).
Fig. 3.
Relative abundance (%) of bacterial groups found in the foam produced by Mahanarva fimbriolata nymphs, in the gut of nymphs, and in the soil close to the foam in a sugarcane field. a Bacterial phyla represented by more than 3% of the sequences. b Bacterial classes represented by more than 2% of the sequences. c Differential relative abundance (%) of bacterial genera in the foam (All genera differed statistically in abundance among the sites sampled, according to analyses using the “edgeR” software package, P < 0.05)
Considering bacterial classes represented by more than 2% of the sequences, Betaproteobacteria (20.5%), Alphaproteobacteria (15.1%), and Flavobacteriia (14.7%) were the most prevalent classes in soil samples, while Alphaproteobacteria (29.9%), Actinobacteria (14.0%), and Chloroacidobacteria (6.3%) were the most prevalent in foam samples. Actinobacteria (18.3%), Thermoleophilia (7.7%), and Betaproteobacteria (6.40%) were the most prevalent in gut samples (Fig. 3b).
Regarding bacterial genera in the spittlebug-derived samples, Bacillus, Streptomyces, Nocardia, and Sphingomonas were the most prevalent genera in foam, while Nitrospira was the most prevalent in gut samples (P < 0.05) (Fig. 3c).
Discussion
The successful dissemination of insects worldwide has been strongly influenced by their associations with microorganisms [14]. While symbiotic microorganisms are well known to play a key role in providing nutrients in many insects, several studies have shown that they can also be important in protecting the host against pathogen attack [17, 18, 40]. Here, we examined the bacterial community present in the foam of M. fimbriolata nymphs, by comparing its abundance and composition with the bacterial communities in the soil, foam, and spittlebug gut. The complete pathway of foam production by cercopids so far is not totally understood. The glands of Batelli were the organs previously believed to produce this substance [1], however some species of the Cercopidae family were able to produce foam without having these organs [41]. Besides, there is evidence of foam production on the food channel [42]. Because of all these contradictory evidences, we decided to evaluate the complete gut of M. fimbriolata, and its bacterial associated community.
Our results revealed the presence of a large number of bacteria in the foam of M. fimbriolata, as previously reported for other spittlebugs [43]. The complexity of the bacterial community present in the foam and its similarity to that in the insect gut suggests that these symbionts are incorporated actively into the spittle. The presence of lipids, carbohydrates, and proteins can make the foam a favorable and beneficial environment with satisfactory nutritional requirements for bacterial growth [5, 6, 43].
Analysis of the bacterial community structure revealed a clear differentiation between foam and soil samples. Also, the similarity between the bacterial communities from the foam and the nymph gut reinforces the supposition that most microorganisms in the foam are not contaminants from the air or soil. The access to food-associated microbial cells, the availability of nutrients, and the protection from various stresses of the external environment are some attributes that make the insect gut favorable for colonization by microorganisms [14].
The means by which insects transmit their symbionts from one generation to another vary widely across different taxonomic groups and individual behavior. In Hemiptera, post-hatch transmission is the most common mechanism of transfer, with symbiont acquisition resulting from ingestion of adult fecal droplets [44], probing capsules containing symbionts deposited close to the eggs [45], or by egg-surface contamination and further probing of symbionts by the nymphs [46].
Our results showed that the foam produced by the nymphs of M. fimbriolata harbors a complex community of microorganisms. We identified 257 specific OTUs in the spittle, including some that were previously reported as defensive symbionts of insects (Figs. 2 and 3). The diversity in the foam was similar to the other microenvironments examined, such as the 288 OTUs in the soil and the 291 OTUs in the insect gut. Actinobacteria was present predominantly in the foam and nymph gut, whereas Proteobacteria was found in all environments, but with higher frequency in the foam than in the gut and soil (Fig. 3a).
The presence of Actinobacteria in the foam and gut of nymphs may be connected to the capacity of these bacteria to exploit a wide range of nutrient sources and their ability to inhabit the intestinal tract of several insects [24, 47, 48]. The ubiquity of this phylum in the environment and their capacity to produce substances with antimicrobial activity has probably predisposed them to be involved in defensive symbioses with soil-dwelling insects [17]. For instance, Streptomyces spp. were found to produce streptochlorin and a complex of eight piericidin derivatives that provide a potent antimicrobial defense for the larval cocoon of P. triangulum wasps in the presence of noxious pathogens [40]. There is also evidence that Proteobacteria promote resistance of insects to certain natural enemies. The Alphaproteobacteria Wolbachia protects Drosophila melanogaster against various viruses [49], and the Betaproteobacteria Regiella insecticola reduces the mortality of pea aphids infected with entomopathogenic fungi [18]. In both cases, the underlying mechanisms are not known.
Since the members of Actinobacteria seem to be rarely involved in nutritional symbiosis with insects [17, 24, 25], we hypothesized that the presence of these bacteria in the foam may aid in protecting against entomopathogenic microorganisms of spittlebug nymphs. However, our current findings do not address this hypothesis, since we did not evaluate the antimicrobial compounds in the foam.
Elucidating the contribution of the resident symbiotic community in the foam to the development of M. fimbriolata nymphs expands our understanding of the ecology of this spittlebug, which is a serious agricultural pest in Brazilian sugarcane crops, and suggests possibilities for biological control by manipulation of the host’s microbiome. Here, we demonstrated that the foam produced by nymphs of M. fimbriolata harbors a diversity of bacteria that were previously reported as protective symbionts of insects. Further investigations on the isolation and identification of substances in the foam can help to understand the possible protective mechanism(s) involved and the presence of antibiotics in the foam.
Electronic supplementary material
(DOCX 19 kb)
Funding information
This work was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001, and the National Institute of Science and Technology – Semiochemicals in Agriculture (Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico [grants #2014/50871-0 and #465511/2014-7, respectively]).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guilbeau BH. The origin and formation of the froth in spittle-insects. Am Nat. 1908;42:783–798. doi: 10.1086/279010. [DOI] [Google Scholar]
- 2.Kato K. The origin and composition of the cuckoo spit. Rept Saitama Univ, B. 1958;3:33–53. [Google Scholar]
- 3.Weaver C, King D (1954) Meadow spittlebug. Ohio Agric Exp Station Res Bull 741:1–99. https://scholar.google.com/scholar_lookup?title=Meadow+spittlebug,+Philaenus+leucophthalmus+(L.)&author=Weaver,+C.R.&author=King,+D.R.&publication_year=1954&journal=Ohio+Agric.+Exp.+Stat.+Bull.&volume=741&pages=1%E2%80%9399
- 4.Marshall AT. Protein synthesis and secretion by the Malpighian tubules of cercopoid larvae (Homoptera) J Insect Physiol. 1973;19:2317–2326. doi: 10.1016/0022-1910(73)90238-2. [DOI] [Google Scholar]
- 5.Mello MLS, Pimentel ER, Yamada AT, Storopoli-Neto A. Composition and structure of the froth of the spittlebug, Deois sp. Insect Biochem. 1987;17:493–502. doi: 10.1016/0020-1790(87)90009-6. [DOI] [Google Scholar]
- 6.Tonelli M, Gomes G, Silva WD, Magri NTC, Vieira DM, Aguiar CL, et al. Spittlebugs produce foam as a thermoregulatory adaptation. Sci Rep. 2018;8:4729. doi: 10.1038/s41598-018-23031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker JB. Cercopid spittle as a microhabitat. Oikos. 1970;21:59–64. doi: 10.2307/3543839. [DOI] [Google Scholar]
- 8.del Campo ML, King JT, Gronquist MR. Defensive and chemical characterization of the froth produced by the cercopid Aphrophora cribrata. Chemoecology. 2011;21:1–8. doi: 10.1007/s00049-010-0059-x. [DOI] [Google Scholar]
- 9.Leite LG, Machado LA, Goulart RM, Tavares FM, Batista FA. Screening of entomopathogenic nematodes (Nemata: Rhabditida) and the efficiency of Heterorhabditis sp. against the sugarcane root spittlebug Mahanarva fimbriolata (Fabr.) (Hemiptera: Cercopidae) Neotrop Entomol. 2005;34:785–790. doi: 10.1590/S1519-566X2005000500010. [DOI] [Google Scholar]
- 10.Dinardo-Miranda LL, Vasconcelos ACM, Vieira SR, Fracasso JV, Grego CR. Uso da geoestatística na avaliação da distribuição espacial de Mahanarva fimbriolata em cana-de-açúcar. Bragantia. 2007;66:449–455. doi: 10.1590/S0006-87052007000300011. [DOI] [Google Scholar]
- 11.Rezende JM, Zanardo ABR, da Silva LM, Delalibera I, Rehner SA. Phylogenetic diversity of Brazilian Metarhizium associated with sugarcane agriculture. BioControl. 2015;60:495–505. doi: 10.1007/s10526-015-9656-5. [DOI] [Google Scholar]
- 12.Ferrari J, Darby AC, Daniell TJ, Godfray HCJ, Douglas AE. Linking the bacterial community in pea aphids with host-plant use and natural enemy resistance. Ecol Entomol. 2004;29:60–65. doi: 10.1111/j.1365-2311.2004.00574.x. [DOI] [Google Scholar]
- 13.Oh D-C, Poulsen M, Currie CR, Clardy J. Dentigerumycin: a bacterial mediator of an ant-fungus symbiosis. Nat Chem Biol. 2009;5:391–393. doi: 10.1038/nchembio.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annu Rev Entomol. 2015;60:17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flórez LV, Biedermann PHW, Engl T, Kaltenpoth M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat Prod Rep. 2015;32:904–936. doi: 10.1039/c5np00010f. [DOI] [PubMed] [Google Scholar]
- 16.Kaltenpoth M, Göttler W, Herzner G, Strohm E. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr Biol. 2005;15:475–479. doi: 10.1016/j.cub.2004.12.084. [DOI] [PubMed] [Google Scholar]
- 17.Kaltenpoth M. Actinobacteria as mutualists: general healthcare for insects? Trends Microbiol. 2009;17:529–535. doi: 10.1016/j.tim.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Scarborough CL, Ferrari J, Godfray HCJ. Aphid protected from pathogen by endosymbiont. Science (80- ). 2005;310:1781 [DOI] [PubMed]
- 19.Mahadav A, Gerling D, Gottlieb Y, Czosnek H, Ghanim M. Parasitization by the wasp Eretmocerus mundus induces transcription of genes related to immune response and symbiotic bacteria proliferation in the whitefly Bemisia tabaci. BMC Genomics. 2008;9:342. doi: 10.1186/1471-2164-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan M, Bharathiraja C, Pandiarajan J, Prasanna VA, Rajendhran J, Gunasekaran P. Insect gut microbiome - an unexploited reserve for biotechnological application. Asian Pac J Trop Biomed. 2014;4(Suppl 1):S16–S21. doi: 10.12980/APJTB.4.2014C95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis Z, Lizé A. Insect behaviour and the microbiome. Curr Opin Insect Sci. 2015;9:86–90. doi: 10.1016/J.COIS.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Douglas AE. Lessons from studying insect symbioses. Cell Host Microbe. 2011;10:359–367. doi: 10.1016/J.CHOM.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucchi TD, Prado SS, Cônsoli FL. The gastric caeca of pentatomids as a house for actinomycetes. BMC Microbiol. 2012;12:101. doi: 10.1186/1471-2180-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem H, Kreutzer E, Sudakaran S, Kaltenpoth M. Actinobacteria as essential symbionts in firebugs and cotton stainers (Hemiptera, Pyrrhocoridae) Environ Microbiol. 2013;15:1956–1968. doi: 10.1111/1462-2920.12001. [DOI] [PubMed] [Google Scholar]
- 26.Moran NA, Tran P, Gerardo NM. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl Environ Microbiol. 2005;71:8802–8810. doi: 10.1128/AEM.71.12.8802-8810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koga R, Bennett GM, Cryan JR, Moran NA. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ Microbiol. 2013;15:2073–2081. doi: 10.1111/1462-2920.12121. [DOI] [PubMed] [Google Scholar]
- 28.Koga R, Moran NA. Swapping symbionts in spittlebugs: evolutionary replacement of a reduced genome symbiont. ISME J. 2014;8:1237–1246. doi: 10.1038/ismej.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scopel W, Cônsoli FL. Culturable symbionts associated with the reproductive and digestive tissues of the neotropical brown stinkbug Euschistus heros. Antonie Van Leeuwenhoek. 2018;:1–12. 10.1007/s10482-018-1130-9 [DOI] [PubMed]
- 30.Muyzer G, de Waal EC, Uitterlinden AG. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/AEM.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1–e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pylro VS, Roesch LFW, Morais DK, Clark IM, Hirsch PR, Tótola MR. Data analysis for 16S microbial profiling from different benchtop sequencing platforms. J Microbiol Methods. 2014;107:30–37. doi: 10.1016/J.MIMET.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 35.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Core Team. R: Language And Environment For Statistical Computing R Foundation for Statistical Computing. 2017
- 37.Ferreira EB, Cavalcanti PP, Nogueira DA. ExpDes: an R package for ANOVA and experimental designs. Appl Math. 2014;05:2952–2958. doi: 10.4236/am.2014.519280. [DOI] [Google Scholar]
- 38.McMurdie PJ, Holmes S. Waste not, want not: why rarefying microbiome data is inadmissible. PLoS Comput Biol. 2014;10:e1003531. doi: 10.1371/journal.pcbi.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhariwal A, Chong J, Habib S, King IL, Agellon LB, Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroiss J, Kaltenpoth M, Schneider B, Schwinger M-G, Hertweck C, Maddula RK, et al. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol. 2010;6:261–263. doi: 10.1038/nchembio.331. [DOI] [PubMed] [Google Scholar]
- 41.Marshall AT. Batelli glands of cercopoid nymphs (Homoptera) Nature. 1965;205:925. doi: 10.1038/205925b0. [DOI] [Google Scholar]
- 42.Cecil R. The alimentary canal of Philaenus leucophthalmus L. Ohio J Sci. 1930;30:120–130. [Google Scholar]
- 43.Wilson HA, Dorsey CK. Studies on the composition and microbiology of insect spittle. Ann Entomol Soc Am. 1957;50:399–406. doi: 10.1093/aesa/50.4.399. [DOI] [Google Scholar]
- 44.Ben BC, Cordon-Rosales C, Durvasula RV. Bacterial symbionts of the triatominae and their potential use in control of Chagas disease transmission. Annu Rev Entomol. 2002;47:123–141. doi: 10.1146/annurev.ento.47.091201.145144. [DOI] [PubMed] [Google Scholar]
- 45.Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 2006;4:e337. doi: 10.1371/journal.pbio.0040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prado SS, Rubinoff D, Almeida RPP. Vertical transmission of a pentatomid caeca-associated symbiont. Ann Entomol Soc Am. 2006;99:577–585. doi: 10.1603/0013-8746(2006)99[577:vtoapc]2.0.co;2. [DOI] [Google Scholar]
- 47.Park D-S, Oh H-W, Jeong W-J, Kim H, Park H-Y, Bae KS. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. J Microbiol. 2007;45:394–401. [PubMed] [Google Scholar]
- 48.Lefebvre T, Miambi E, Pando A, Diouf M, Rouland-Lefèvre C. Gut-specific actinobacterial community structure and diversity associated with the wood-feeding termite species, Nasutitermes corniger (Motschulsky) described by nested PCR-DGGE analysis. Insect Soc. 2009;56:269–276. doi: 10.1007/s00040-009-0020-6. [DOI] [Google Scholar]
- 49.Hedges LM, Brownlie JC, O’Neill SL, Johnson KN. Wolbachia and virus protection in insects. Science. 2008;322:702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 19 kb)