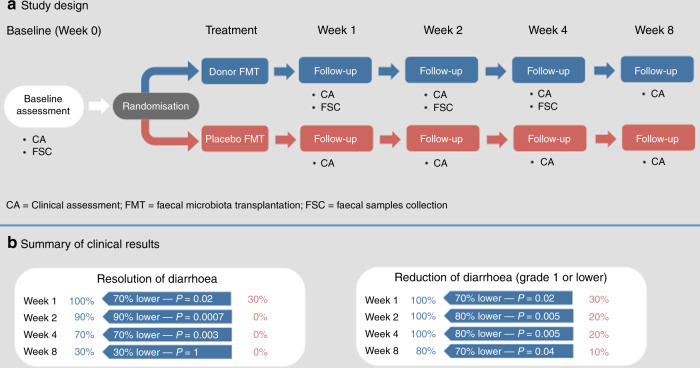
Fig. 1. Study design and summary of clinical results.
a Study design: after baseline clinical and microbiome assessments, patients were randomised to receive either donor faecal microbiota transplantation (D-FMT) or placebo FMT (P-FMT). Then, all patients were followed up to 8 weeks (follow-up visits at 1, 2, 4 and 8 weeks after treatments). b Summary of clinical results: D-FMT was significantly more effective than P-FMT in achieving the complete resolution of TKI-induced diarrhoea at 1 week (n = 10 vs n = 3, 100% vs 30%, p = 0.02), 2 weeks (n = 9 vs n = 0, 90% vs 0%, p = 0.0007), 4 weeks (primary outcome, n = 7 vs n = 0, 70% vs 0%, p = 0.003), but not at 8 weeks of follow-up (n = 3 vs n = 0, 30% vs 0%, p = 1), consistently with the single FMT infusion approach of the study. Nonetheless, the benefit of D-FMT over P-FMT in decreasing diarrhoea up to G1 grade or lower remained significant for the whole follow-up period, including 1 week (n = 10 vs n = 3, 100% vs 30%, p = 0.02), 2 weeks (n = 10 vs n = 2, 100% vs 20%, p = 0.005), 4 weeks (n = 10 vs n = 2, 100% vs 20%, p = 0.005) and 8 weeks (n = 8 vs n = 1, 80% vs 10%, p = 0.04) after the end of treatments. Differences in cure percentages were determined with Fisher’s exact test (with two-tailed p values). The Bonferroni adjustment was applied for multiple secondary outcomes.