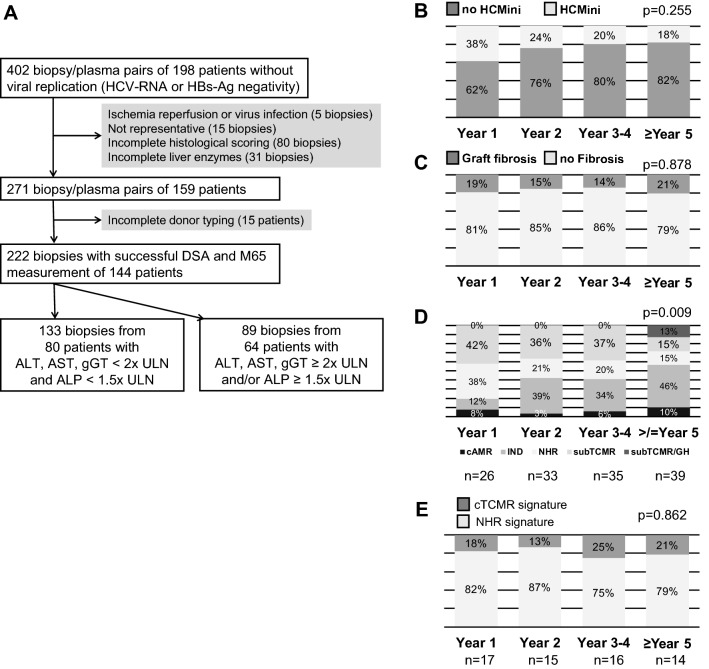
Figure 1.
Biopsy selection and time course of subclinical histological findings. (A) Flow chart outlining availability and selection of biomaterial for this study. (B–E) Prevalence of histological findings (histological criteria for minimization of immunosuppression (HCMini) and at least moderate graft fibrosis), diagnosis (cAMR = possible chronic antibody-mediated rejection; IND = indeterminate findings; NHR = no histological rejection; subTCMR = subclinical T cell-mediated rejection; GH = graft hepatitis not fulfilling cAMR criteria) and graft gene expression signatures (cTCMR = clinical relevant T cell mediated rejection) in liver biopsies with normal/marginally elevated liver enzymes (n = 133) over time. P-values of Chi2 test of the prevalence of time are depicted.