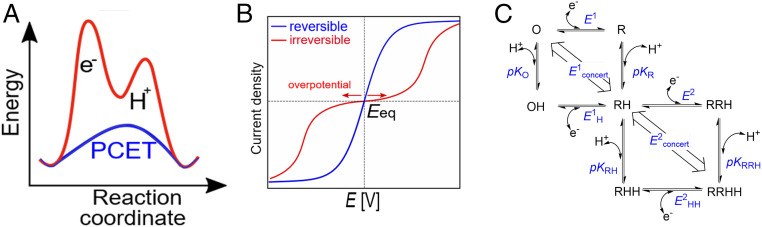
Fig. 2.
(A) General reaction scheme, showing the energetic advantage of PCET compared to uncoupled proton and ET steps. (B) Ideal waveshapes in cyclic voltammetry that are proposed to describe reversible (blue) and irreversible (red) electrocatalysts (adapted from ref. 30). (C) Square scheme in which horizontal sides are redox couples defined by electron-only reduction potentials and the diagonal lines are the redox couples defined by the reduction potential for concerted PCET. O/R: oxidized/reduced state. E1/E1H: reduction potential of the unprotonated/protonated state. E2/E2HH: reduction potential of the reduced, onefold protonated/reduced, twofold protonated state. pKO/pKR: pK of the oxidized/reduced state. pKRH/pKRRH: pK of the onefold protonated onefold/twofold reduced state.