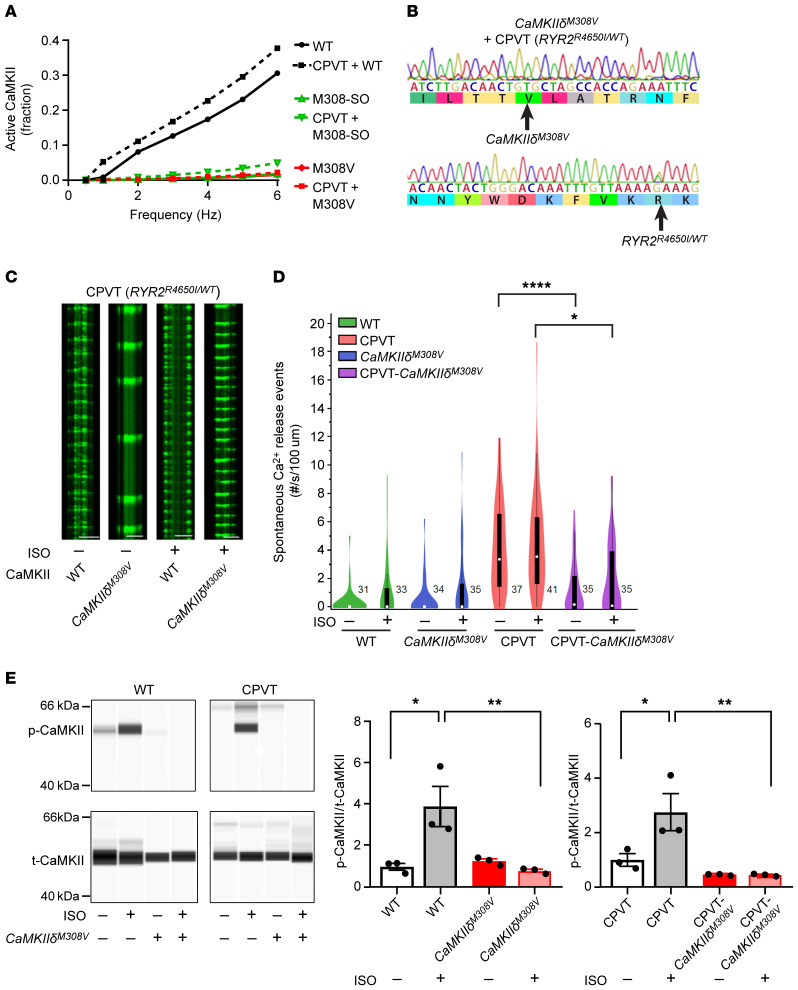
Figure 6. Prevention of CaMKII hyperactivation by M308V rescues catecholaminergic polymorphic ventricular tachycardia (CPVT).
(A) Modeling comparing WT, M308-SO, and M308V CaMKII holoenzyme activity under normal and CPVT conditions. Note that M308-SO and M308V curves under normal and CPVT conditions are superimposed. (B) Successful introduction of M308V (CaMKIIδM308V) in iPSCs from patients with CPVT (RyR2R4650I/WT) was confirmed with Sanger sequencing. (C) Representative confocal line scans of hiPSC-derived cardiomyocytes expressing GCaMP6f-Junctin nanosensor at baseline and after isoproterenol (ISO) treatment. Horizontal scale bars: 20 μm. (D) Spontaneous Ca2+ release events measured with the GCaMP6f-Junctin nanosensor in hiPSC-derived cardiomyocytes from controls (WT), patients with CPVT (CPVT), and their isogenic lines with homozygous CaMKIIδM308V. Recordings at baseline and after isoproterenol treatment (WT n = 31–33 cells, WT-CaMKIIδM308V n = 34–35 cells, CPVT n = 37–41 cells, CPVT-CaMKIIδM308V n = 35 cells). (E) Left: Western blots showing autophosphorylated-T287 CaMKII (p-CaMKII) and total CaMKII (t-CaMKII) in WT and CPVT hiPSC–derived cardiomyocytes with WT or CaMKIIδM308V at baseline and after isoproterenol treatment. Quantification is shown in right panels (n = 3 in each group). *P < 0.05; **P < 0.01; ****P < 0.0001 by 1-way ANOVA with Tukey’s multiple-comparisons test (D and E).