Abstract
Background
Men who have sex with men (MSM) are at risk for sexual transmission of enteric pathogens. The microbiology of gastroenteritis in MSM has not been examined since the advent of antiretroviral therapy and molecular diagnostics. Our objective was to assess the causes of gastroenteritis among MSM living with and without human immunodeficiency virus (HIV) coinfection in Seattle, Washington.
Methods
We conducted a retrospective cohort study of 235 MSM who underwent multiplex stool polymerase chain reaction (PCR) testing between 1 January 2017 and 1 June 2018. We abstracted clinical and laboratory data from electronic medical records. Parallel or reflexive culture and susceptibility testing were performed when PCR detected cultivable pathogens.
Results
Among 235 MSM tested (268 episodes), 131 had 151 episodes with positive test results. 148 (63.0%) individuals were living with HIV. Among positive tests, 88.7% detected a bacterial pathogen, 26% a virus, and 40% a parasite. Diarrheagenic Escherichia coli (enteroaggretative, enteropathogenic), Shigella, and Campylobacter were the most commonly detected bacteria (33.1%, 30.5%, and 17.2% of positive samples, respectively). Forty-three percent of positive specimens had ≥2 pathogens. Etiologies and clinical presentations were similar between men living with and without HIV. Cultured Shigella and Campylobacter isolates were frequently resistant to multiple antibiotics.
Conclusions
MSM present with gastroenteritis from varied pathogens, including some not detected by conventional stool culture. High levels of antibiotic resistance are consistent with frequent antibiotic exposure in this population and the transmission of multiresistant strains. New approaches are needed to detect, treat, and prevent enteric infections in MSM.
Keywords: acute gastroenteritis, infectious diarrhea, men who have sex with men, antimicrobial resistance, HIV
Men who have sex with men have high prevalence of bacterial, parasitic, and viral gastroenteritis, including multidrug-resistant pathogens. Molecular diagnostics can facilitate pathogen detection in this population, but improved surveillance, prevention, and treatment of sexually transmitted enteric infections are needed.
Prior to the human immunodeficiency virus (HIV) epidemic, men who have sex with men (MSM) with multiple sexual partners were recognized to have a higher prevalence of intestinal pathogens, in particular, Shigella, Campylobacter, and Giardia, than the general population, [1–3]. This led to the recognition that enteric pathogens can be spread not only via traditional water- and foodborne routes but also through sexual activity [1]. Outbreaks of sexually transmitted Shigella infections have been increasingly documented throughout the world [4–10], and MSM and international travelers are recognized as high-risk groups for antibiotic-resistant Shigella. HIV infection compounds the risk of enteric infection by enhancing host susceptibility to opportunistic pathogens such as Salmonella and Cryptosporidium [11, 12].
Several studies have described the range of pathogens responsible for enteric infections in MSM. Such studies have focused largely on proctitis and proctocolitis [3, 13–15] and have mostly been conducted in sexually transmitted disease (STD) clinics with populations enriched for patients who present with classic sexually transmitted infections (eg, Chlamydia, gonorrhea, syphilis) using traditional diagnostic methods that predate the development of highly sensitive culture-independent molecular diagnostic tests [16, 17]. The present study was undertaken to examine the etiology of infectious diarrhea in MSM in the era of molecular diagnostics.
METHODS
Patient Population
Multiplex polymerase chain reaction (PCR) testing using a commercial panel was implemented for general stool pathogen testing in January 2017 across the University of Washington healthcare system including the University of Washington Medical Center, Seattle Cancer Care Alliance, Harborview Medical Center, and the University of Washington–affiliated outpatient clinics. A previously reported prospective multicenter observational study that involved these clinical sites was performed from 1 January 2017 to 30 September 2017 [18]. Based on the results of the initial analysis, the study was extended to focus on the subset of MSM patients with the study period extended until 1 June 2018.
Laboratory Evaluation
Stool specimens submitted for diagnostic testing were transported in Cary-Blair transport medium and tested using the FilmArray GI Panel (BioFire Diagnostics, Salt Lake City, UT) upon receipt or on the following morning for specimens received between 11 pm and 7 am. From 1 January 2017 to 30 September 2017, stool cultures using blood, MacConkey, MacConkey-sorbitol, Salmonella-Shigella selective, cefsulodin-irgasan-novobiocin, and Campylobacter selective agar were performed in parallel. Thiosulfate-citrate-bile salt-sucrose agar to enhance the recovery of Vibrio spp. was added from July to September. Subsequently, targeted cultures were performed for specimens with cultivable pathogens detected using the GI Panel.
Chart Review
A retrospective cohort analysis of the patient subset with a sex marker of male who were aged ≥18 years at the time of testing was performed. Chart review was used to identify patients who were MSM, defined as having documented sexual contact with male partners, a male intimate partner, or being described as “gay” or “homosexual” in the medical chart. For patients identified as MSM who underwent stool PCR testing with the FilmArray GI Panel, an in-depth chart review was performed to capture demographic information, presenting symptoms, HIV status, antiretroviral medication (ARV) use, additional diagnostic testing performed (white blood cell count; stool ova and parasite examination; testing for Cryptosporidium, Cyclospora, and Cystoisospora by modified acid-fast smear; Chlamydia and gonorrhea testing by PCR and culture; syphilis testing by serum rapid plasma reagin), antimicrobial treatment, and resolution of symptoms. All authors have doctoral-level training in clinical medicine or microbiology and participated in chart review.
Comparison Group
MSM data were compared to contemporaneous records from non-MSM individuals who underwent gastrointestinal pathogen testing using the same laboratory methods at the same institutions (previously published by Cybulski et al [18]). Individuals identified as MSM were excluded from the data from Cybulski et al to create Figure 1 and Supplementary Figure 1.
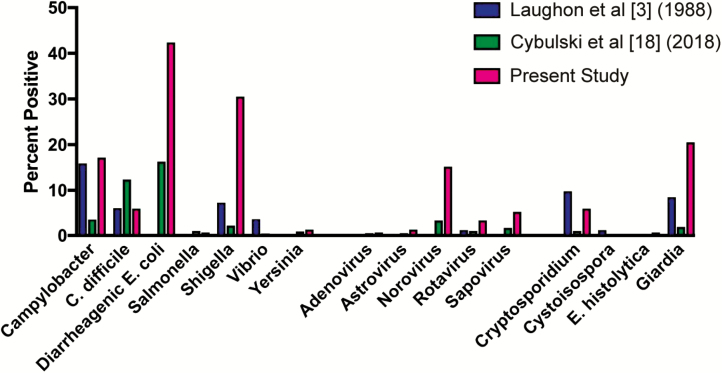
Figure 1.
Pathogens detected by stool testing from episodes of gastroenteritis. The figure compares historical data from men who have sex with men in Baltimore, Maryland ([3]; n = 85, 85% living with human immunodeficiency virus [HIV]), contemporary data from the total University of Washington patient population in Seattle ([18]; n = 669), and this study (n = 151, 75% living with HIV).
Data Analyses
Clinical variables between men living with and without HIV were compared, and significance was determined using the Student t test, χ2 test, Fisher exact test, or Wilcoxon rank sum as appropriate. A P value of <.05 was considered significant. Statistical analyses were performed in R (R Core Team 2018, Vienna, Austria).
Ethics Statement
The University of Washington Institutional Review Board approved the study.
RESULTS
Demographics
A total of 235 MSM with symptoms of gastroenteritis were tested during the study period, of whom 130 (57.5%) had 1 or more clinical encounter for which they had a positive stool enteric pathogen test. A total of 151 clinical encounters had positive test results. The demographic features of individuals who underwent testing for diarrheal pathogens are described in Table 1. Overall, the majority (66%) of the population was non-Hispanic white, 11% were black, and 13% were Hispanic. While 64.6% of episodes involved individuals living with HIV, 70% of positive tests were from clinical encounters that involved individuals living with HIV, of which 82% were collected while the individual was on ARV therapy. Of positive tests from clinical encounters involving individuals living without HIV, more than 60% were collected while the individual was on preexposure prophylaxis (PrEP). Nearly 70% of testing occurred in the outpatient setting and an additional 15% in the emergency department. Concurrent testing for gonorrhea, chlamydia, and syphilis occurred less than one-third of the time.
Table 1.
Demographics and Clinical Characteristics of Men Who Have Sex With Men With Stool Pathogen Testing (N = 268 Episodes) and Comparisons for Differences Between Episodes Involving Individuals Living With and Without Human Immunodeficiency Virus With Positive Stool Pathogen Testing
| Characteristic | Overall (N = 268) | Pathogen-Positive Episode (n = 151) | ||
|---|---|---|---|---|
| HIV+ | HIV− | |||
| n = 100 | n = 51 | P Value | ||
| Age, mean (standard deviation), y | 42.6 (13.5) | 43.7 (11.6) | 33.0 (12.9) | <.001 |
| Race/ethnicity | .38 | |||
| White non-Hispanic | 178 (66.4) | 70 (70.0) | 34 (66.7) | |
| Black | 30 (11.2) | 11 (11.0) | 6 (11.8) | |
| Hispanic | 34 (12.7) | 10 (10.0) | 5 (9.8) | |
| Asian | 14 (5.2) | 3 (3.0) | 5 (9.8) | |
| Other | 12 (4.5) | 6 (6.0) | 1 (2.0) | |
| Location of testinga | .05 | |||
| Inpatient | 39 (14.6) | 12 (12.0) | 3 (5.9) | |
| Outpatient | 186 (69.4) | 63 (63.0) | 42 (82.4) | |
| Emergency department | 40 (14.9) | 25 (25.0) | 6 (11.8) | |
| Additional testing | ||||
| White blood cell count | 149 (55.6) | 63 (63.0) | 26 (51.0) | .21 |
| Stool ova and parasites exam | 97 (36.2) | 36 (36.0) | 18 (35.3) | .99 |
| Cryptosporidium, Cyclospora, and Isospora (Cystoisospora) | 48 (17.9) | 22 (22.0) | 8 (15.7) | .35 |
| Neisseria gonorrhoeae /Chlamydia | 59 (22.0) | 30 (30.0) | 14 (27.5) | .89 |
| Syphilis | 54 (20.1) | 28 (28.0) | 13 (25.5) | .89 |
| Preexposure prophylaxis | 36 (13.4) | NA | 31 (60.8) | … |
| Antiretroviral medications | 139 (51.9) | 82 (82.0) | NA | … |
| CD4, median (IQR) | 375 (187–648) | 444 (187–697.5) | NA | |
| Viral load | ||||
| Undetectable, n (%) | 112 (41.8) | 61 (61.0) | NA | … |
| Detectable, median (IQR) | 50 220 (1262.5–209 350) | 32 865 (554.5–359 000) | NA | … |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable.
aThree observations missing location of testing.
Symptoms
Presenting symptoms and history are listed in Table 2. Overall, there was little difference observed in the clinical presentation of individuals living with and without HIV with gastroenteritis and positive test results. Individuals living with HIV were more likely to have diarrhea, especially watery diarrhea. Individuals living without HIV were significantly more likely to report a history of recent travel. For both groups, in nearly 14% of episodes with positive testing, the diarrhea was described as bloody, but tenesmus was rare (6%). Temperature >38ºC (12.5%), subjective fever or chills (24.5%), or leukocytosis (14.5%) were reported in a minority of episodes with positive testing.
Table 2.
Clinical Characteristics of Men Who Have Sex With Men During Episodes of Gastroenteritis With Stool Pathogen Testing (N = 268 Episodes) and Comparisons for Differences Between Episodes Involving Individuals Living With and Without Human Immunodeficiency Virus With Positive Stool Pathogen Testing (all n, % Unless Otherwise Noted)
| Characteristic | Pathogen-Positive Episode (n = 151) | |||
|---|---|---|---|---|
| Overall | HIV+ | HIV− | ||
| N = 268 | n = 100 | n = 51 | P Value | |
| Diarrhea | 239 (89.2) | 98 (98.0) | 41 (80.4) | <.001 |
| Watery | 120 (44.8) | 54 (54.0) | 16 (31.3) | .01 |
| Bloody | 29 (10.8) | 14 (14.0) | 7 (13.7) | .99 |
| Nausea | 83 (31.0) | 35 (35.0) | 17 (33.3) | .99 |
| Vomiting | 46 (17.2) | 22 (22.0) | 7 (13.7) | .28 |
| Abdominal pain | 142 (53.0) | 63 (63.0) | 32 (62.7) | .99 |
| Tenesmus | 13 (4.9) | 7 (7.0) | 2 (3.9) | .78 |
| Headache | 18 (6.7) | 8 (8.0) | 5 (9.8) | .76 |
| Fever/chills | 56 (20.9) | 25 (25.0) | 12 (23.5) | .99 |
| Fatigue | 55 (20.5) | 24 (24.0) | 9 (17.6) | .53 |
| Symptom duration, median (interquartile range), days | 10 (4–30) | 7 (3–18) | 7 (4–21) | .20 |
| Sick contact | 20 (7.5) | 11 (11.0) | 5 (9.8) | .99 |
| Travel | 27 (10.0) | 6 (6.0) | 12 (23.5) | <.01 |
| Fever (>38ºC) | 37 (13.8) | 15 (15.0) | 6 (11.8) | .99 |
| Leukocytosis (>10K white blood cells) | 27 (10.0) | 13 (13.0) | 7 (13.7) | .99 |
Abbreviation: HIV, human immunodeficiency virus.
Microbiology
Overall, 65 (43%) samples were positive for 2 or more potential pathogens (Table 3). Twenty-nine percent of tests were positive for 2 pathogens, 9% for 3 pathogens, and 5% for 4 or more. Of all positive test results, 88.7% detected a bacterial pathogen, while 26% detected a virus and 40% detected a parasite. Of the bacterial causes, diarrheagenic Escherichia coli, in particular, enteroaggretative E. coli (EAEC) and enteropathogenic E. coli (EPEC), was most common, representing 33% of the pathogens detected (Figure 1, Supplementary Table 1). Shigella species comprised 30% and Campylobacter species 17%. Norovirus was the most common viral pathogen identified, representing 59% of viruses and 15% of pathogens overall. Giardia was the most common parasite identified, comprising 50% of parasitic pathogens and 20.5% of pathogens overall. Of samples in which more than 1 pathogen was found, diarrheagenic E. coli and Shigella predominated. Limited differences were observed between encounters that involved patients living with and without HIV. Shigella was more common in patients living with HIV (36% vs 20% of detected pathogens, P = .04). In contrast, Giardia was more common in patients living without HIV than in patients living with HIV (33% vs 14%, respectively; P < .01). Among patients living with HIV, Cryptosporidium was identified more commonly in those with a CD4 count <200 (1% vs 17.8%, data not shown), although there was no significant difference between patients living with and without HIV in aggregate.
Table 3.
Incidence of Multiple Pathogen Detection on Stool Testing, Ancillary Testing, Antibiotic Use, and Clinical Outcomes of Men Who Have Sex With Men With Gastroenteritis and Positive Pathogen Stool Testing (N = 151 Episodes)
| Characteristic | Overall | HIV+ | HIV− | |
|---|---|---|---|---|
| N = 151 | n = 100 | n = 51 | P Value | |
| Pathogens detected by multiplex polymerase chain reaction, mean (standard deviation) | 1.6 (0.9) | 1.7 (0.9) | 1.6 (0.9) | .61 |
| Number of pathogens detected (%) | .74 | |||
| 1 | 86 (57.0) | 56 (56.0) | 30 (58.8) | |
| 2 | 44 (29.1) | 29 (29.0) | 15 (29.4) | |
| 3 | 14 (9.3) | 9 (9.0) | 5 (9.8) | |
| >4 | 8 (5.3) | 6 (6.0) | 1 (2.0) | |
| Additional pathogens detected, n (%) | ||||
| Gonorrhea | 6 (4.0) | 4 (4.0) | 2 (3.9) | .99 |
| Chlamydia | 9 (6.0) | 7 (7.0) | 2 (3.9) | .71 |
| Acute syphilis | 4 (2.6) | 4 (4.0) | 0 (0.0) | .30 |
| Received antimicrobial, n (%) | 115 (75.7) | 75 (75.0) | 40 (76.9) | .84 |
| Duration of treatment, median (IQR), days | 5 (3–7) | 5 (3–7) | 3 (3–7) | .07 |
| Length of stay,a median (IQR), days | 4 (2–5.25) | 4 (2–6) | 3 (3-3) | .31 |
| Clinical change, n (%) | .09 | |||
| Resolved | 112 (73.7) | 74 (74.0) | 38 (73.1) | |
| Improved | 18 (11.9) | 16 (16.0) | 2 (3.8) | |
| Unchanged | 11 (7.2) | 7 (7.0) | 4 (7.7) | |
| Unknown | 8 (5.3) | 3 (3.0) | 5 (9.6) | |
| Otherb | 2 (1.3) | 0 (0.0) | 2 (3.8) | |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
an = 25 admitted to the hospital overall, 19 HIV+, 6 HIV−.
bIncludes an individual who died (n = 1) individual who was asymptomatic when diagnosed (n = 1).
Response to Treatment
In encounters with positive testing, resolution of symptoms was documented in 112, with only 11 episodes resulting in unchanged symptoms (Table 3). Overall, antibiotics were given in 75% of episodes with positive testing. The most common antibiotics prescribed were ciprofloxacin and azithromycin, which were prescribed in 40.9% and 21.7%, respectively, of cases in which antibiotics were prescribed. The mean duration of antibiotic treatment was 5 days. Fourteen patients (13%) did not receive antibiotics despite the detection of a bacterial pathogen, and 11 (79%) of them experienced spontaneous resolution of their symptoms. For 27 patients with Campylobacter or Shigella with available resistance testing, 2 did not receive antibiotics and 4 received antibiotics to which their isolate was resistant. Despite this, all experienced resolution or improvement of their symptoms.
Antimicrobial Resistance
Of 19 Shigella isolates tested, all were resistant to ampicillin and trimethoprim-sulfamethoxazole, and all but 2 were resistant to azithromycin (Supplementary Figure 1). Shigella isolates were generally sensitive to ciprofloxacin based on Clinical and Laboratory Standards Institute criteria of minimum inhibitory concentration (MIC) <1 µg/mL, with only 1 of 19 tested demonstrating high-level resistance (MIC >2 µg/mL). The Shigella isolate that was resistant to ciprofloxacin remained sensitive to azithromycin. However, only 2 of 10 Shigella flexneri isolates had a ciprofloxacin MIC ≤0.12 µg/mL. In contrast, all but 1 of 9 isolates of Shigella sonnei had ciprofloxacin MIC <0.12 µg/mL. Nine Campylobacter isolates were tested for antimicrobial susceptibility. Seven (78%) were resistant to ciprofloxacin (MICs 8 to >256 µg/mL), and 8 (89%) were resistant to erythromycin (MIC >256 µg/mL). Resistance testing was not performed for azithromycin, and the single isolate that was tested against doxycycline was resistant (MIC 64 µg/mL).
DISCUSSION
This is the largest study of the causes of gastroenteritis in MSM and the first such study in the HIV era to use comprehensive molecular diagnostic testing. Specific pathogens were detected in 56.3% of cases, a substantially higher diagnostic yield than the 33.5% yield observed in the general population [18]. Our observations confirm earlier studies that indicated that MSM are at higher risk for infectious diarrhea caused by Campylobacter, Shigella, and Giardia and extend the known microbial spectrum of gastroenteritis in MSM to include diarrheagenic E. coli, in particular, EAEC and EPEC, and norovirus. Clostridium difficile was detected less frequently in MSM than in the general population.
Shigella or diarrheagenic E. coli were detected in more than one-half of the positive fecal specimens. This stands in contrast to studies of the etiology of acute gastroenteritis in the general population in high-resource settings. Among patients who presented to 9 emergency departments in the United States, norovirus was the most common pathogen (26%), and bacteria collectively were responsible for only 17% of cases [19]. Studies in Austria, the Netherlands, and Germany similarly found bacterial pathogens to account for a minority of cases of acute gastroenteritis [20–22]. While these studies were completed prior to the advent of culture-independent diagnostics and thus had lower sensitivity, they remain the only studies of the etiology of gastroenteritis in large, primary care populations. At our institutions over the same time period as the present study, MSM had higher rates of diarrheagenic E. coli (42.4 vs 16.3%), Shigella (30.5% vs 2.2%), Giardia (20.5% vs 1.9%), and Campylobacter (17.2% vs 3.6%) in comparison to the general population [18] (Figure 1).
The increased risk of Shigella in MSM [1, 3, 23] and the sexual transmission of Shigella in outbreaks [4–9, 24–27] has been well documented. Recent studies in Australia that used whole-genome sequencing have provided evidence of ongoing transmission of related clusters of S. flexneri and S. sonnei among MSM [28]. The prevention of sexually transmitted enteric pathogens may require different considerations than the control of foodborne outbreaks that involve the same pathogens. Although antibiotic therapy is selectively administered to patients with foodborne gastroenteritis depending on the severity of symptoms [29], it may play a more prominent role in arresting transmission via sexual exposure. The potential utility of screening asymptomatic high-risk individuals for enteric pathogen colonization must also be considered. The high proportion of individuals with Shigella who were using PrEP correlates with increasing rates of bacterial sexually transmitted infections in persons using PrEP [30] and underscores emerging evidence that risk compensation by PrEP users may be leading to more high-risk sexual practices [31]. Although living with HIV is a risk factor for severity and complications of bacterial enteritis [11, 12, 32], we did not observe significant differences in the clinical presentations of MSM living with and without HIV, which likely reflects the effectiveness of contemporary antiretroviral therapy.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization have declared antimicrobial-resistant Shigella to represent a major public health threat [33, 34]. A 2017 CDC advisory suggested that Shigella spp. with ciprofloxacin MIC of 0.12–1.0 µg/mL may not be suitable for treatment with ciprofloxacin [35], despite being categorized as susceptible by current interpretive criteria. We observed generally higher rates of antimicrobial resistance in bacterial isolates from MSM compared to men who do not have sex with men (Supplementary Figure 1). Shigella flexneri and S. sonnei isolates from MSM were almost uniformly resistant to azithromycin and also resistant to ampicillin and trimethoprim-sulfamethoxazole. Along with other reports, this suggests the rapid emergence of azithromycin resistance in Shigella spp. from MSM [28, 36–38]. Of 15 S. flexneri isolates from MSM, 12 had a ciprofloxacin MIC of 0.12–1.0 µg/mL. All of these patients had resolution of their symptoms after receipt of ciprofloxacin, suggesting that fluoroquinolones may still be clinically effective for strains with MICs in this range. Antibiotic-resistant Campylobacter is also a global health priority [33]. Although the number of Campylobacter isolates available for susceptibility testing in our study was limited, high rates of resistance to macrolides and/or fluoroquinolones in Campylobacter coli and Campylobacter jejuni are of concern and have been previously noted in MSM-related outbreaks [39, 40]. High rates of resistance in enteric pathogens from MSM may reflect both the transmission of multidrug-resistant outbreak strains and selective pressure from frequent antibiotic exposure. Oral treatment options for Shigella and Campylobacter spp. that are resistant to both fluoroquinolones and macrolides are limited.
Furthering concerns about antibiotic resistance is the high rate of prescription of antibiotics in our study regardless of detected etiology. Clinicians in our study prescribed antibiotics in 75.7% of visits with positive testing. Infectious Disease Society of America (IDSA) guidelines recommend empiric antibiotics in immunocompetent adults who show signs and symptoms of bacillary dysentery in order to treat presumptive shigellosis [41]. However, Shigella was detected in only 30% of positive tests. Furthermore, IDSA guidelines recommend testing only in cases of suspected bacillary dysentery or moderate to severe watery diarrhea. We suspect that the high rate of testing and treatment in our study is due to 2 factors. First, many consider people living with HIV to be immunocompromised regardless of CD4 count and may therefore test and treat people living with HIV based on immunocompromised guideline recommendations. Second, there is active discussion in the STD community regarding testing and treating of gastroenteritis in MSM [42], with some advocating for increased testing and treatment in MSM to prevent further transmission.
The strengths of our study include the use of sensitive molecular microbiological diagnostic testing, a relatively large cohort for which detailed clinical information was available, a study population who presented for routine medical care, and the availability of susceptibility testing for Shigella and Campylobacter isolates. Limitations include a patient population from a single healthcare system that may not necessarily be representative of MSM populations elsewhere, the lack of susceptibility testing for uncultured bacterial isolates, and a reliance on retrospective clinical data from chart review. An important limitation of our study is inherent to PCR-based testing, which is highly sensitive and able to detect both colonized and infected individuals. This may be particularly important for diarrheagenic E. coli, which is commonly found in asymptomatic carriers, but Campylobacter and Shigella may also be carried asymptomatically [43–45]. This underscores the importance of appropriate patient selection for testing and treatment [41, 46]; most patients in our study had symptoms consistent with acute gastroenteritis. It is possible that some of the organisms in patients with multiple targets detected were not responsible for the patients’ symptoms.
In conclusion, MSM are at increased risk for gastroenteritis from a broad range of pathogens including diarrheagenic E. coli, Giardia, norovirus, and multidrug-resistant Shigella and Campylobacter spp. In the era of effective antiretroviral therapy, the clinical presentation and outcomes of enteric infections in individuals living with and without HIV appears to be comparable. However, antimicrobial treatment options are becoming limited, and persistent evidence of transmission through sexual contact suggests that current prevention strategies are ineffective. New approaches are needed to detect, treat, and prevent enteric infections in MSM.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the University of Washington and Harborview Medical Center clinical microbiology laboratories for their assistance with the execution of this study.
Potential conflicts of interest. F. C. F. reports grants, personal fees, and nonfinancial support from BioFire during the conduct of the study. F. C. F. also reports grants, personal fees, and nonfinancial support from Cepheid; grants and nonfinancial support from ELITech; nonfinancial support from Luminex; and personal fees from the Infectious Diseases Society of America outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dritz SK, Ainsworth TE, Back A, et al. . Patterns of sexually transmitted enteric diseases in a city. Lancet 1977; 2:3–4. [DOI] [PubMed] [Google Scholar]
- 2. Forthal DN, Guest SS. Isospora belli enteritis in three homosexual men. Am J Trop Med Hyg 1984; 33:1060–4. [DOI] [PubMed] [Google Scholar]
- 3. Laughon BE, Druckman DA, Vernon A, et al. . Prevalence of enteric pathogens in homosexual men with and without acquired immunodeficiency syndrome. Gastroenterology 1988; 94:984–93. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention. Shigella sonnei outbreak among men who have sex with men—San Francisco, California, 2000–2001. MMWR Morb Mortal Wkly Rep 2001; 50):922–6. [PubMed] [Google Scholar]
- 5. Marcus U, Zucs P, Bremer V, et al. . Shigellosis—a re-emerging sexually transmitted infection: outbreak in men having sex with men in Berlin. Int J STD AIDS 2004; 15:533–7. [DOI] [PubMed] [Google Scholar]
- 6. Morgan O, Crook P, Cheasty T, et al. . Shigella sonnei outbreak among homosexual men, London. Emerg Infect Dis 2006; 12:1458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okame M, Adachi E, Sato H, et al. . Shigella sonnei outbreak among men who have sex with men in Tokyo. Jpn J Infect Dis 2012; 65:277–8. [DOI] [PubMed] [Google Scholar]
- 8. Bowen A, Eikmeier D, Talley P, et al. . Notes from the field: outbreaks of Shigella sonnei infection with decreased susceptibility to azithromycin among men who have sex with men—Chicago and Metropolitan Minneapolis–St. Paul, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:597–8. [PMC free article] [PubMed] [Google Scholar]
- 9. Valcanis M, Brown JD, Hazelton B, et al. . Outbreak of locally acquired azithromycin-resistant Shigella flexneri infection in men who have sex with men. Pathology 2015; 47:87–8. [DOI] [PubMed] [Google Scholar]
- 10. Hines JZ, Pinsent T, Rees K, et al. . Notes from the field: shigellosis outbreak among men who have sex with men and homeless persons—Oregon, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65:812–3. [DOI] [PubMed] [Google Scholar]
- 11. Jacobs JL, Gold JW, Murray HW, Roberts RB, Armstrong D. Salmonella infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1985; 102:186–8. [DOI] [PubMed] [Google Scholar]
- 12. Payne P, Lancaster LA, Heinzman M, McCutchan JA. Identification of Cryptosporidium in patients with the acquired immunodeficiency syndrome. N Engl J Med 1983; 309:613–4. [DOI] [PubMed] [Google Scholar]
- 13. Quinn TC, Corey L, Chaffee RG, Schuffler MD, Brancato FP, Holmes KK. The etiology of anorectal infections in homosexual men. Am J Med 1981; 71:395–406. [DOI] [PubMed] [Google Scholar]
- 14. Klausner JD, Kohn R, Kent C. Etiology of clinical proctitis among men who have sex with men. Clin Infect Dis 2004; 38:300–2. [DOI] [PubMed] [Google Scholar]
- 15. Mitchell H, Hughes G. Recent epidemiology of sexually transmissible enteric infections in men who have sex with men. Curr Opin Infect Dis 2018; 31:50–6. [DOI] [PubMed] [Google Scholar]
- 16. Buss SN, Leber A, Chapin K, et al. . Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol 2015; 53:915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Binnicker MJ. Multiplex molecular panels for diagnosis of gastrointestinal infection: performance, result interpretation, and cost-effectiveness. J Clin Microbiol 2015; 53:3723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cybulski RJ Jr, Bateman AC, Bourassa L, et al. . Clinical impact of a multiplex gastrointestinal polymerase chain reaction panel in patients with acute gastroenteritis. Clin Infect Dis 2018; 67:1688–96. [DOI] [PubMed] [Google Scholar]
- 19. Bresee JS, Marcus R, Venezia RA, et al. ; US Acute Gastroenteritis Etiology Study Team The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis 2012; 205:1374–81. [DOI] [PubMed] [Google Scholar]
- 20. de Wit MA, Koopmans MP, Kortbeek LM, et al. . Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol 2001; 154:666–74. [DOI] [PubMed] [Google Scholar]
- 21. Jansen A, Stark K, Kunkel J, et al. . Aetiology of community-acquired, acute gastroenteritis in hospitalised adults: a prospective cohort study. BMC Infect Dis 2008; 8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huhulescu S, Kiss R, Brettlecker M, et al. . Etiology of acute gastroenteritis in three sentinel general practices, Austria 2007. Infection 2009; 37:103–8. [DOI] [PubMed] [Google Scholar]
- 23. Tauxe RV, McDonald RC, Hargrett-Bean N, Blake PA. The persistence of Shigella flexneri in the United States: increasing role of adult males. Am J Public Health 1988; 78:1432–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bader M, Pedersen AH, Williams R, Spearman J, Anderson H. Venereal transmission of shigellosis in Seattle–King county. Sex Transm Dis 1977; 4:89–91. [DOI] [PubMed] [Google Scholar]
- 25. Strauss B, Kurzac C, Embree G, Sevigny R, Paccagnella A, Fyfe M. Clusters of Shigella sonnei in men who have sex with men, British Columbia, 2001. Can Commun Dis Rep 2001; 27:109–10; discussion 10–4. [PubMed] [Google Scholar]
- 26. O’Sullivan B, Delpech V, Pontivivo G, et al. . Shigellosis linked to sex venues, Australia. Emerg Infect Dis 2002; 8:862–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gaudreau C, Ratnayake R, Pilon PA, Gagnon S, Roger M, Levesque S. Ciprofloxacin-resistant Shigella sonnei among men who have sex with men, Canada, 2010. Emerg Infect Dis 2011; 17:1747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ingle DJ, Easton M, Valcanis M, et al. . Co-circulation of multidrug-resistant Shigella among men who have sex with men, Australia. Clin Infect Dis 2019. Epub ahead of print. 10.1093/cid/ciz005 [DOI] [PubMed] [Google Scholar]
- 29. Williams PCM, Berkley JA. Guidelines for the treatment of dysentery (shigellosis): a systematic review of the evidence. Paediatr Int Child Health 2018; 38:50–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beymer MR, DeVost MA, Weiss RE, et al. . Does HIV pre-exposure prophylaxis use lead to a higher incidence of sexually transmitted infections? A case-crossover study of men who have sex with men in Los Angeles, California. Sex Transm Infect 2018; 94:457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Traeger MW, Schroeder SE, Wright EJ, et al. . Effects of pre-exposure prophylaxis for the prevention of human immunodeficiency virus infection on sexual risk behavior in men who have sex with men: a systematic review and meta-analysis. Clin Infect Dis 2018; 67:676–86. [DOI] [PubMed] [Google Scholar]
- 32. Angulo FJ, Swerdlow DL. Bacterial enteric infections in persons infected with human immunodeficiency virus. Clin Infect Dis 1995; 21(Suppl 1):S84–93. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics Available at: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/. Accessed 26 January.
- 34.Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. 2013. Atlanta, GA: CDC. Available at: https://emergency.cdc.gov/han/han00401.asp [Google Scholar]
- 35. CDC recommendations for diagnosing and managing Shigella strains with possible reduced susceptibility to Ciprofloxacin. HAN 2017; 401. [Google Scholar]
- 36. Bowen A, Grass J, Bicknese A, Campbell D, Hurd J, Kirkcaldy RD. Elevated risk for antimicrobial drug-resistant Shigella infection among men who have sex with men, United States, 2011–2015. Emerg Infect Dis 2016; 22:1613–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chiou CS, Izumiya H, Kawamura M, et al. . The worldwide spread of ciprofloxacin-resistant Shigella sonnei among HIV-infected men who have sex with men, Taiwan. Clin Microbiol Infect 2016; 22:383 e11–6. [DOI] [PubMed] [Google Scholar]
- 38. Murray K, Reddy V, Kornblum JS, et al. . Increasing antibiotic resistance in Shigella spp. from infected New York City residents, New York, USA. Emerg Infect Dis 2017; 23:332–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaudreau C, Pilon PA, Sylvestre JL, Boucher F, Bekal S. Multidrug-resistant Campylobacter coli in men who have sex with men, Quebec, Canada, 2015. Emerg Infect Dis 2016; 22:1661–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gaudreau C, Michaud S. Cluster of erythromycin- and ciprofloxacin-resistant Campylobacter jejuni subsp. jejuni from 1999 to 2001 in men who have sex with men, Quebec, Canada. Clin Infect Dis 2003; 37:131–6. [DOI] [PubMed] [Google Scholar]
- 41. Shane AL, Mody RK, Crump JA, et al. . 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis 2017; 65:1963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pakianathan M. Should enteric infections in MSM always be treated? In: ISSTDR 2019. Vancouver, Canada, 2019.
- 43. Platts-Mills JA, Liu J, Rogawski ET, et al. . Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 2018; 6:e1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Platts-Mills JA, Babji S, Bodhidatta L, et al. . Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bruijnesteijn van Coppenraet LE, Dullaert-de Boer M, Fuijs GJ, et al. . Case-control comparison of bacterial and protozoan microorganisms associated with gastroenteritis: application of molecular detection. Clin Microbiol Infect 2015; 21:592.e9–19. [DOI] [PubMed] [Google Scholar]
- 46. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 2016; 111:602–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.