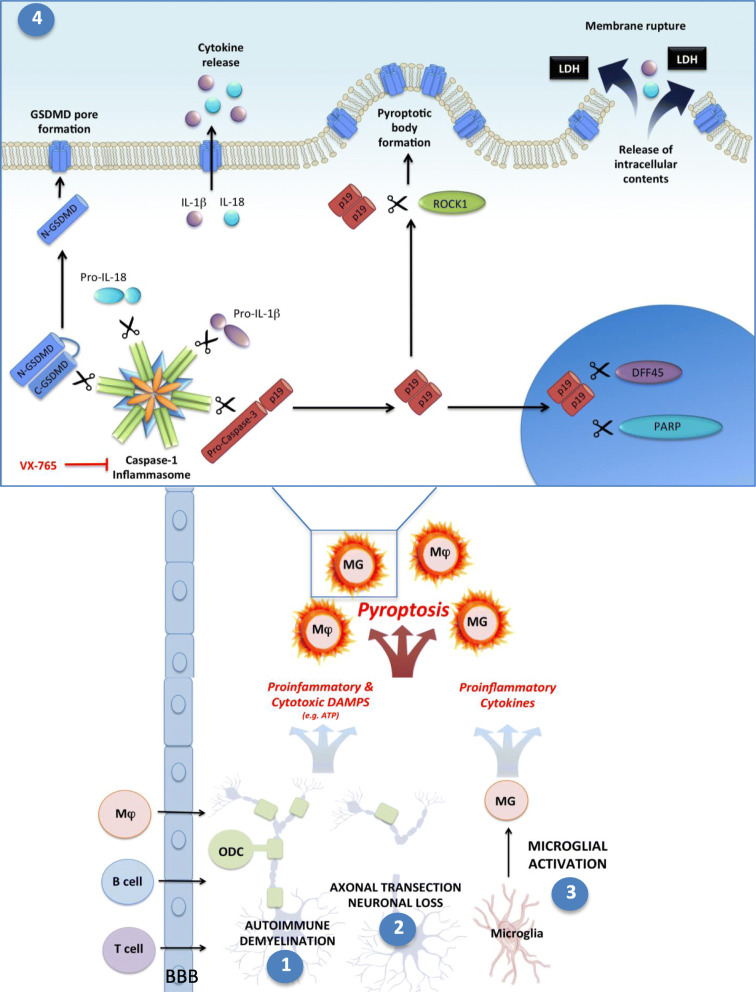
Fig. 9.
Activated caspases-3 and -7 contribute to pyroptosis in macrophage/microglia in multiple sclerosis. MS neuropathogenesis is driven by converging disease processes that create a proinflammatory microenvironment, including infiltration of peripheral immune cells, demyelination (1), axonal loss and neuronal cell death (2), and microglial activation (3). Widespread cell death releases proinflammatory DAMPs and alarmins (e.g., extracellular ATP), which trigger pyroptosis in macrophages (Mφ)/microglia (MG). Pyroptosis is lytic and highly proinflammatory, creating a positive feedback loop to drive further pyroptotic cell death. Within the pyroptotic cell (4), inflammasome activation leads to the cleavage and activation of proinflammatory cytokines (IL-1β and IL-18) and the cytotoxic pore-forming protein GSDMD, and as shown in this manuscript, procaspase-3. While activated GSDMD translocates to the plasma membrane to form pores, cleaved caspase-3 traffics to multiple cellular compartments wherein it cleaves several substrates that promote pyroptosis. In the nucleus, caspase-3 and -7 substrates DFF45 and PARP are cleaved and activated. ROCK1 is cleaved and activated in the cytoplasm and appears to concentrate around the cell periphery and in pyroptotic bodies, suggesting a putative role in pyroptotic body formation that mimics its role in apoptotic body formation during apoptosis. Collectively, the proteolytic network engaged by caspase-3/7 during pyroptosis activates parallel processes that facilitate cellular demolition, pyroptotic body formation, and membrane lysis during pyroptosis