Figure 3.
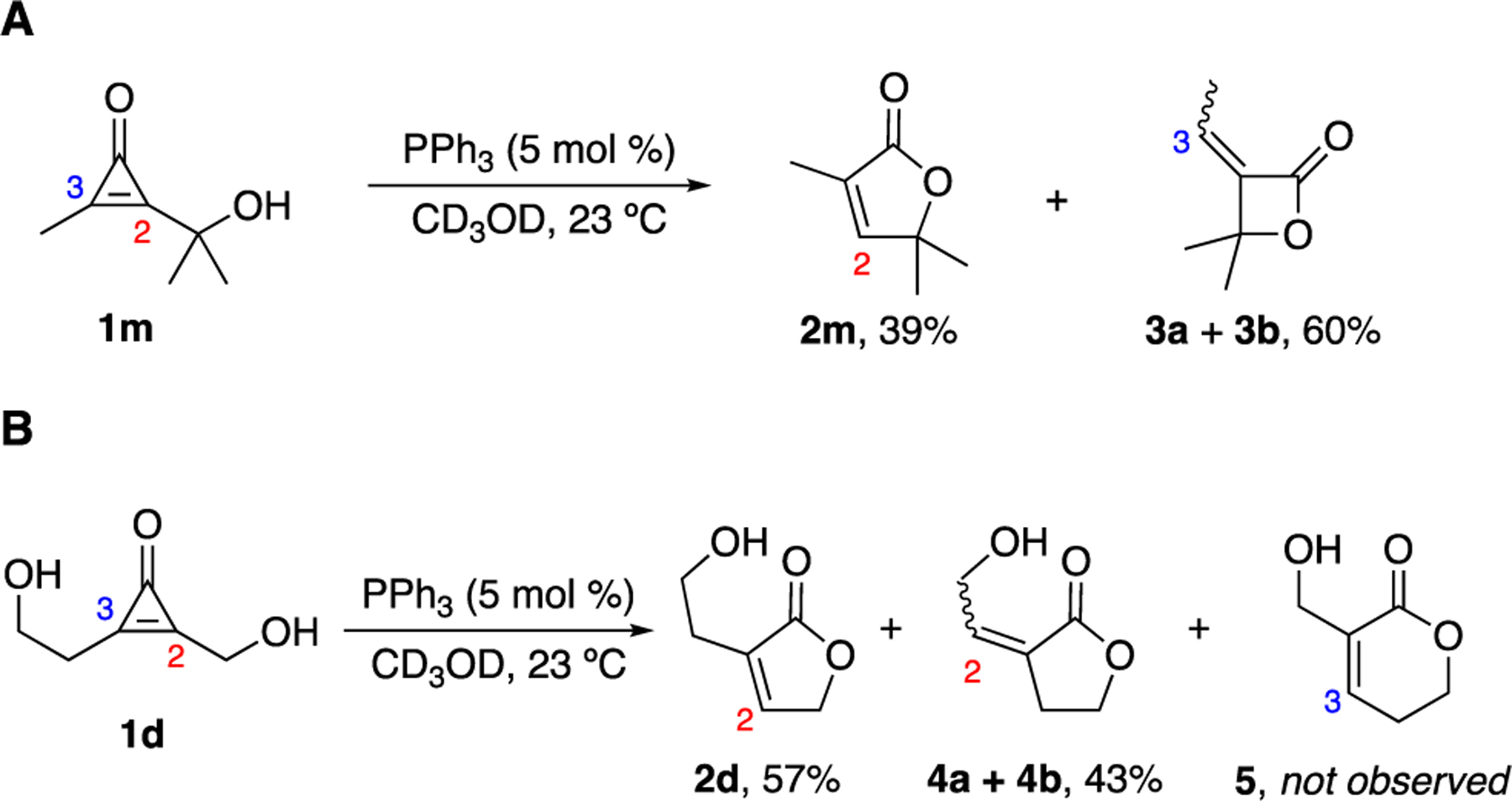
Mechanistic studies involving butenolide formation. (A) Upon phosphine treatment, CpO 1m formed β-lactone products 3a-b. The additional steric bulk at C2 likely disfavored phosphine addition. (B) δ-Lactone 5 was not observed when diol-CpO 1d was treated with PPh3. This reaction produced a mixture of γ-lactones (4a-b), in addition to the desired butenolide 2d. For (A)-(B), percent conversion values (from NMR analyses) are reported.
