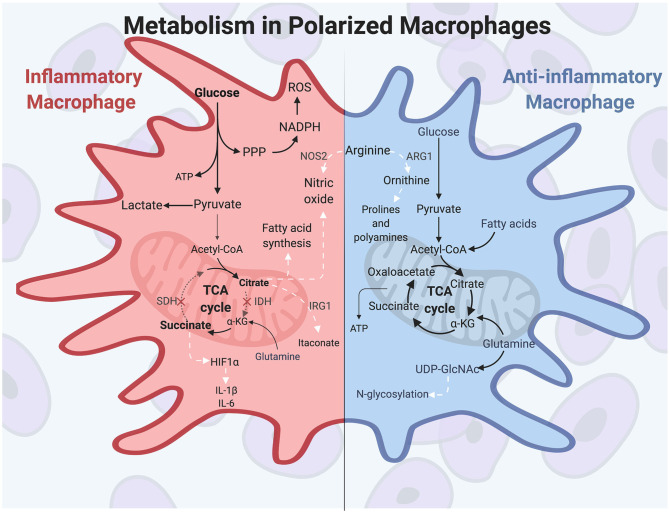
Figure 1.
Polarized macrophages have differential metabolic programming. Inflammatory macrophages characteristically increase glucose uptake to fuel aerobic glycolysis, producing increased lactate and ATP production. The pentose phosphate pathway feeds off of the increased flux of glycolytic intermediates, resulting in heightened nucleotide and amino acid synthesis. Classically activated macrophages have a disrupted TCA cycle in two locations: at isocitrate dehydrogenase (IDH) and at succinate dehydrogenase (SDH). As a result, citrate and succinate accumulate and drive such functions as fatty acid and NO synthesis, antimicrobial itaconate production, and HIF1α activity to promote further glycolysis and inflammatory cytokine production. Arginine is differentially metabolized in polarized murine macrophages, producing the antimicrobial molecule NO in inflammatory macrophages. Anti-inflammatory macrophages are not as metabolically active compared to classically activated macrophages, and they utilize glutamine, glucose, and fatty acids to fuel the TCA cycle and OXPHOS. In alternatively activated macrophages, glutamine uptake drives UDP-GlcNAc production, which is important for N-glycosylation of cell surface receptors, such as CD206 and CD301. In anti-inflammatory murine macrophages, arginine is metabolized to ornithine, which promotes tissue repair through production of prolines and polyamines. White arrows indicate metabolic intermediates driving M1 or M2 activities. α-KG, alpha-ketoglutarate; ARG1, arginase; HIF1α, hypoxia-inducible factor 1α; IDH, isocitrate dehydrogenase; IRG1, immune-responsive gene 1; NOS2, nitric oxide synthase; PPP, pentose phosphate pathway; ROS, reactive oxygen species; SDH, succinate dehydrogenase.