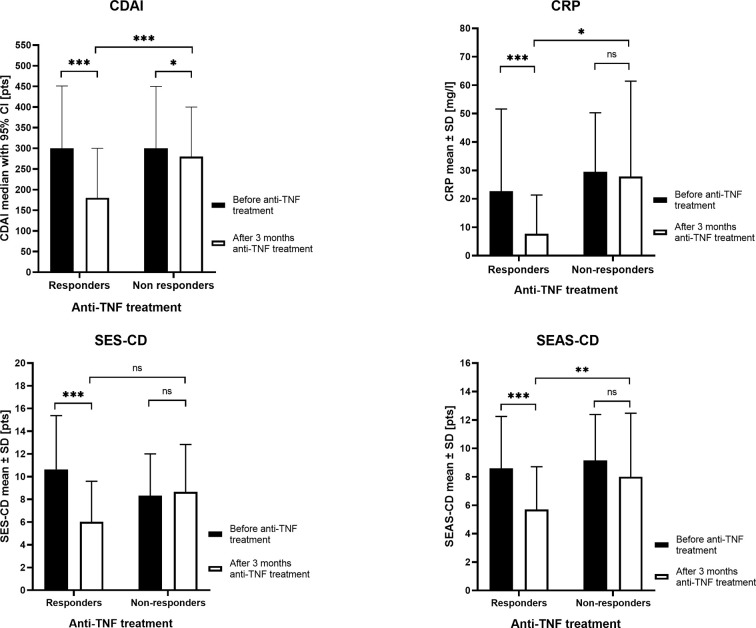
Figure 1.
The clinical parameters in responders and nonresponders before anti-tumor necrosis factor (anti-TNF) treatment and after three months of therapy. CDAI, Crohn’s Disease Activity Index; CRP, C Reactive Protein; SES-CD, Simple Endoscopic Score for Crohn’s Disease; SEAS-CD, Simple Enterographic Activity Score for Crohn’s Disease; CI, confidence interval; SD, standard deviation; ns, not significant; *p < 0.05, **p < 0.01, ***p < 0.001.