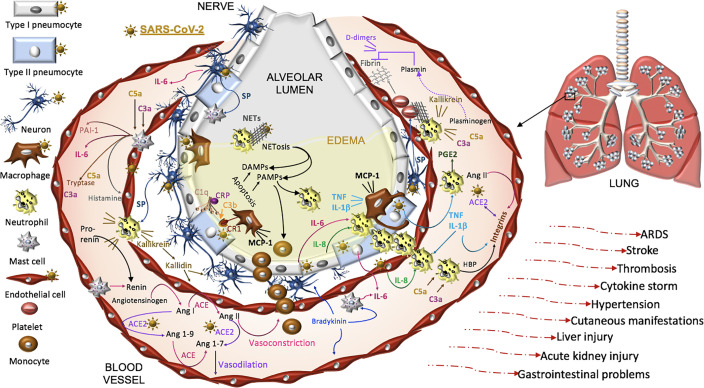
Figure 3.
Proposed model of SARS-CoV-2 pathogenesis. SARS-CoV-2 binds ACE2 on alveolar type I and type II cells, macrophages, neurons, and arterial and venous endothelial cells. Complement, damage-and pathogen-associated molecular pattern ligands (DAMPs, PAMPs), and cytokines prime circulating neutrophils that are recruited in response to IL-8 and IL-6 released from infected cells and mast cells. SARS-CoV-2-induces epithelial apoptosis. Alveolar macrophages remove apoptotic cells through complement independent and dependent mechanisms. Apoptotic cells generate antigens that bind C1q and the interaction may be enhanced by C reactive protein (CRP). The activation of C1q induces C3b deposition for macrophage complement receptor 1 (CR1) binding and phagocytosis. Activated neutrophils produce heparin binding protein (HBP), prostaglandins (PGE2), and extracellular traps (NETs) to capture and kill the virus. NET activity induces a form of cell death called NETosis. Both NETosis and apoptosis generate DAMPs and PAMPs that bind and activate toll-like receptors in promoting inflammation. Mast cells and neutrophils are activated by complement factors and the neuropeptide, substance P (SP), which promotes their degranulation. Mast cells also produce histamine that promotes vasodilation, tryptase involved in complement factor production, and renin in the RAAS. Excessive inflammation, associated with MCP-1-recruited monocytes, promotes the accumulation of fluid, leading to alveolar edema. Platelets are activated by SP and exhibit cross-talk with neutrophil NETs in promoting coagulation. The kallikrein-kinin system is activated by damaged tissue and cells, such as neutrophils. Kallikrein functions as a precursor to bradykinin, activates pro-renin, and cleaves complement C3 and C5 as well as plasminogen. The latter generates plasmin involved in the degradation of fibrin and the formation of D-dimers identified in COVID-19 patient serum. SARS-CoV-2-induced degradation of ACE2 promotes RAAS activity, vasoconstriction, and hypertension. Angiotensin II, various cytokines, and HBP induce the expression of endothelial integrins. In the absence of ACE2, which also binds integrins, the functions of integrins may be dysregulated, promoting inflammation, hypertension, and thromboses. The infection can proceed to acute respiratory distress syndrome (ARDS) and culminates in additional tissues and organs in response to systemic infection.