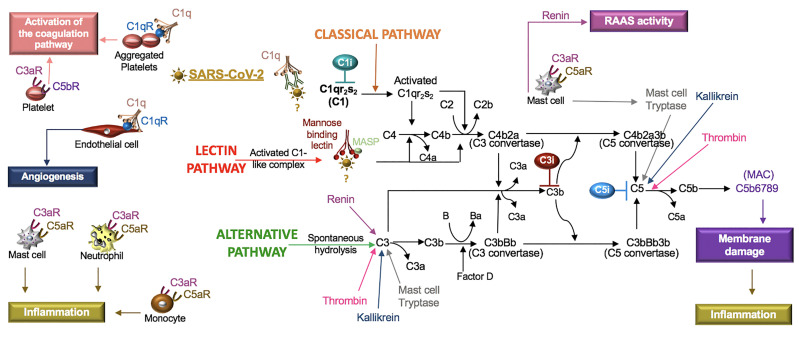
Figure 5.
SARS-CoV-2 in the complement system. Classical, lectin, and alternative are the three pathways in the complement system. Complement components C1, C2, C3, and C4 are present in plasma in inactive forms. In the classical pathway, the C1 component, C1q, recognizes apoptotic cells directly or pathogens indirectly through antibody complexes or associations with pentraxins. In the lectin pathway, mannose-binding lectin (MBL) binds the surface of the pathogen. Serine proteases complex with C1q (C1r/C1s) and MBL (MASP: MBL-associated serine protease), which leads to cleavage of C4 to its fragments (C4b and C4a) and the formation of a C3 and C5 convertase. In the alternative pathway, C3 is spontaneously hydrolyzed and through the activity of factor D forms a C3 convertase and subsequently a C5 convertase. Mast cell tryptase, thrombin or kallikrein can also cleave C3 and C5 whereas renin cleaves only C3. Cleavage fragments from these pathways (e.g. C3a, C5a, C5b) activate immune cell subsets to produce inflammation or coagulation. The terminal product of these pathways, C5b6789, is a membrane attack complex (MAC), that creates a pore in cell membranes by displacing phospholipids. The resulting cell lysis induces inflammatory responses. C1q also acts independent of the complement system and binds its receptor (C1qR) on aggregated platelets and endothelial cells in the promotion of coagulation and angiogenesis, respectively. Tissue and organ damage and excessive inflammation in some COVID-19 patients may indicate that SARS-CoV-2 activates the complement cascade. C1, C3, and C5 inhibitors (i) block factor formation in the complement cascade.