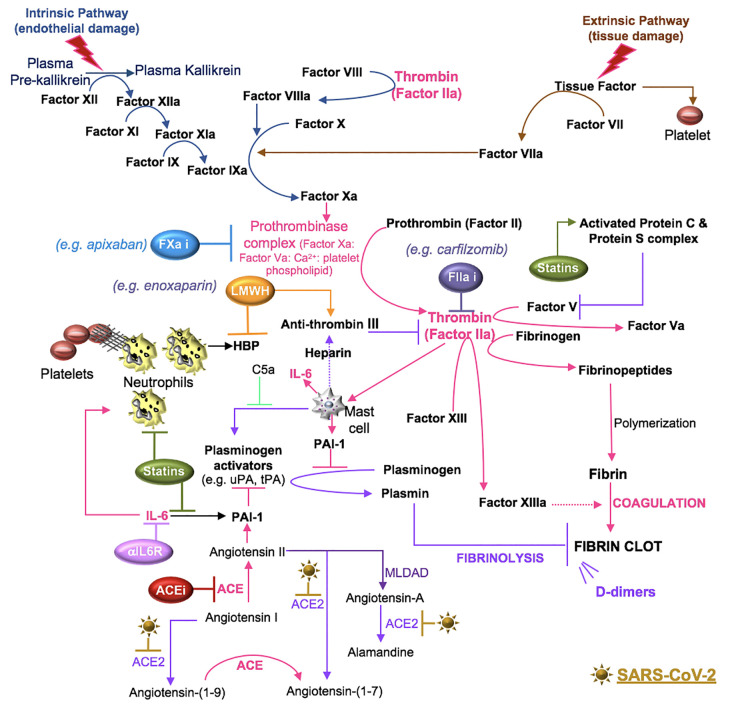
Figure 6.
SARS-CoV-2 in coagulation. Primary hemostasis controls platelet aggregation and secondary hemostasis promotes fibrin formation through the clotting cascade, the latter involving intrinsic and extrinsic pathways. In the intrinsic pathway, damage-induced release of endothelial collagen activates factor XII (factor XIIa), which is a reaction that is also involved in the initiation of plasma kallikrein. Factor XIIa acts as a catalyst to activate factor XI to factor XIa. Factor XIa activates factor IX to factor IXa and the latter, acting with factor VIIIa as a cofactor, activates factor X to factor Xa. Tissue injury releases tissue factor into the blood, which activates platelets to induce neutrophil extracellular trap (NET) formation. The components of NETs reciprocally activate platelets and their aggregation. In the extrinsic pathway, tissue factor activates factor VII (factor VIIa), which activates factor X (factor Xa). The common coagulation pathway commences at factor X, with factor Xa, factor Va, calcium and platelet phospholipids forming the prothrombinase complex, which activates prothrombin (factor II) to thrombin (factor IIa). Thrombin cleaves factor V, VIII, factor XIII and fibrinogen. Polymerization of the formed fibrinopeptides produces fibrin, which is covalently cross-linked by FXIIIa to form a stable nascent fibrin clot. These processes can be antagonized by the heparin-dependent activity of antithrombin and plasmin degradation of fibrin into soluble fibrin degradation products (e.g. D-dimer). Plasmin formation is reduced by angiotensin II- or IL-6-induced plasminogen activator inhibitor (PAI)-1, which antagonizes the activity of urokinase plasminogen activator (uPA) and tissue plasminogen activator (tPA). SARS-CoV-2-induced degradation of ACE2 reduces ACE2 cleavage of angiotensin I and II and the anti-inflammatory effects of the ACE2 fragment [Ang-(1-7)], suggesting that inhibitors of RAAS [e.g. ACE inhibitor (ACEi)] may counter the effects of SARS-CoV-2. Mast cells activated by thrombin or complement (C5a) also contribute to coagulation through the preferential production of IL-6 and PAI-1. Drugs that inhibit IL-6 signals (e.g. anti-IL-6 receptor antibodies) may inhibit IL-6-induced PAI-1 production and IL-6 recruitment of neutrophils. Statins also inhibit IL-6 production, the activity of neutrophils and the functions of activated protein C. The use of inhibitors (i) to the clotting cascade such as factor Xa (e.g. apixaban) and factor IIa (e.g. carfilzomib) may impede SARS-CoV-2-induced coagulation responses. Lastly, low molecular weight heparin (LMWH) therapy may promote the effects of anti-thrombin and inhibit the functions of the alarmin, heparin binding protein (HBP), which binds glycosaminoglycan moieties of cell surface proteoglycans and promotes endothelial permeability.