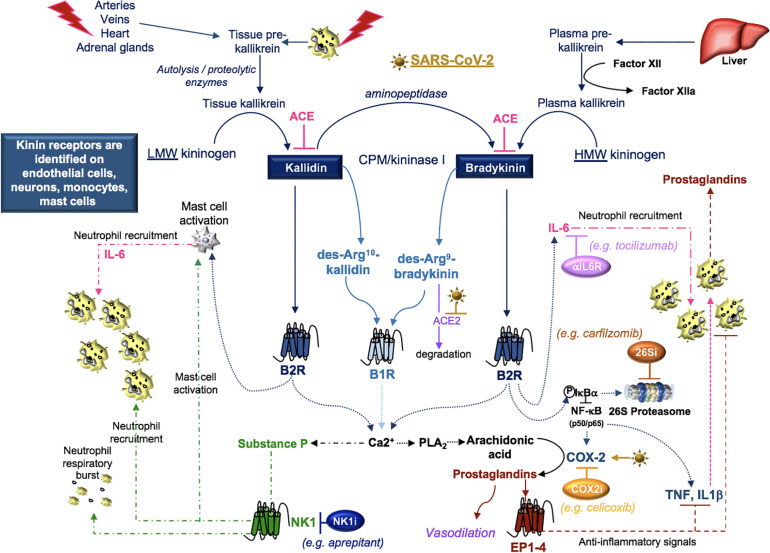
Figure 7.
SARS-CoV-2 and kinins. Tissue prekallikrein released from neutrophils and additional cell types is cleaved by cell surface proteases into tissue kallikrein, which promotes the transformation of low molecular weight (LMW) kininogen into kallidin. An aminopeptidase cleaves kallidin and generates bradykinin, which is a product of factor IIa-activated plasma kallikrein and high molecular weight (HMW) kininogen. Kallidin and bradykinin can be cleaved by ACE (kininase II) to form inactive peptides or carboxypeptidase-M (CPM/kininase I), which forms bradykinin 1 receptor (B1R) ligands des-Arg10-kallidin and des-Arg9-bradykinin. ACE2 is the SARS-CoV-2 receptor, suggesting that ACE2 degradation of des-Arg9-bradykinin may be impaired in COVID-19 patients. Both kallidin and bradykinin activate the bradykinin receptor, B2R, which induces the activation of mast cells, the phosphorylation/degradation of IkBα through the 26S proteasome and the release of NF-kB transcription factors involved in the production of cytokines and the prostaglandin-generating enzyme COX-2. Proteasome inhibitors (26Si) antagonize NF-kB activation. B1R and B2R induce a calcium flux that promotes substance P production and the activation of phospholipase A2 (PLA2) enzymes. PLA2 liberates arachidonic acid for COX-2-induced production of prostaglandins which can be blocked with COX-2 inhibitors (COX2i). Prostaglandins induce anti-inflammatory signals. Substance P and bradykinin-induced IL-6 recruit neutrophils into tissues. Substance P also promotes the degranulation of mast cells and neutrophils. Neurokinin-1 (NK1) receptor antagonist (NKi) may block neutrophil recruitment and respiratory burst activity.