Abstract
Opioids are essential first line analgesics for pain management after burn injury. Opioid dosing remains challenging in burn patients, particularly in children, due to the immense variability in efficacy between patients. Opioid pharmacokinetics are altered in burned children, increasing variability and obviating dosing regimens extrapolated from adult-data. The present study aimed to characterize variability in fentanyl pharmacokinetics and identify significant contributors to variability in children with ≥10% total body surface area burn requiring fentanyl during routine wound care. We recorded patient demographics and clinical data. Blood samples were collected following fentanyl administration for pharmacokinetics at time 0, 30, 60, 120, and 240 minutes on day of admission and repeated on days 3 and 7. Serum fentanyl concentrations were quantified using tandem liquid chromatography mass spectrometry. Population analysis was used to estimate pharmacokinetics parameters. Fourteen patients, 1.2–17 years, with burns from 10–50.5% were included in analysis. A two-compartment model with body weight as a covariate best described fentanyl pharmacokinetics for the overall population. The population clearance and intercompartmental clearance were 7.19 and 2.16 L/hour, respectively, and the volume of distribution for the central and peripheral compartments was 4.01 and 25.1 L, respectively. Individual patient parameter estimates had extensive variability. This study confirmed the high variability in pediatric burn patient fentanyl pharmacokinetics and demonstrates similarities and differences to other populations reported in literature. Further research is needed with a larger number of patients to extensively investigate the impact of burns, genetic polymorphisms, and other factors on fentanyl efficacy and patient outcomes.
Burn injury results in significant pain which is difficult to manage, particularly in children. Severe burn injury requires intense management for both the prolonged pain associated with burn injury and short episodes of acute pain associated with procedures such as dressing changes.1 Opioids are the cornerstone for pain management in burn patients because the opioids have superior efficacy in providing sufficient analgesia. Fentanyl, a synthetic opioid frequently used for burn patient and ICU patient management, was synthesized beginning in the 1950s, to enhance both efficacy and potency, whereas decreasing adverse effects compared with meperidine and morphine.1–4 Fentanyl has been extensively studied for over 50 years with numerous publications determining the pharmacokinetic (PK) parameters for specific populations, including but not limited to critically ill adults,5 geriatrics,6 oncology,7 pediatrics,8,9 and neonates.10
There is extensive variability in fentanyl PK7,11—leading to a wide range of intra-and inter-patient variability. Factors influencing fentanyl PK include administration of drugs that inhibit or induce drug metabolizing pathways (eg, CYP3A4 enzymes), impaired organ function (eg, hepatic), and patient age (eg, geriatrics, neonates).7,10 Burn injury in particular creates physiological conditions affecting drug absorption, distribution, metabolism, and elimination (ADME).12 Volume of distribution (Vd), for instance, is altered by fluid resuscitation, severe blood loss and transfusions, and extreme alterations in body weight with resulting alterations to body mass index which are all common during burn critical care. Moreover, organ dysfunction and hypermetabolism can drastically affect drug ADME.7,12 Infections, including both bacterial and fungal, require administration of drugs with inducing or inhibitory effects on the same metabolizing pathways that opioids use (eg, CYP3A4 inhibition by antifungals).13 Burn patients, specifically those with larger burns, receive an average of 40 different drugs during hospitalization, many of which may affect opioid PK and pharmacodynamics (PD).14
The need to better understand fentanyl PK/PD is more important than ever. The traditional dosing practice of “one size fits all” has resulted in poor pain management for many individuals, whereas others have experienced toxic and life-threatening adverse reactions. As the era of precision medicine is upon us, we must provide evidence based rational when developing individual dosing regimens from data specific for that given population. The current study aims to characterize fentanyl PK and identify significant covariates affecting PK variability in pediatric burn patients receiving fentanyl sedation for dressing changes.
METHODS
Patient Enrollment
This prospective observational study, approved by our institution’s human subjects review board, enrolled children (age ≤18 years) with burns ≥10% total body surface area (TBSA). Other inclusion criteria include: 1) requiring fentanyl therapy for pain management, and 2) having existing vascular access for blood sampling. Exclusion criteria include: 1) nonsurvivable injuries and/or 2) condition where collection of research samples is unsafe (eg, severe anemia).
Sample and Data Collection
Patient demographic data, including age, weight, and ethnicity, were recorded from the electronic medical record. Additionally, history of drug use if available, other medical conditions, as well as vital signs and routine laboratory results, procedures (eg, surgery and line placement) and all drug administrations, including doses and route, from the patient’s medical record were recorded into a HIPPA compliant (eg, deidentification) electronic research database (OnCore, Forte Research Systems, Madison, WI) and all HIPAA guidelines (eg, deidentification) were followed. Patients were dosed with fentanyl either as a single bolus or two to three small boluses over a brief period of time. Upon initiation of fentanyl therapy on day of admission, five blood samples at time 0 (immediately following dosing), 30, 60, 120, and 240 minutes were collected for fentanyl assays. Whole blood was collected from indwelling lines, then immediately centrifuged to yield serum, and stored at −80°C for batch testing. The blood collection series was repeated on days 3 and 7 at the time of dressing change if fentanyl was administered.
Data Analysis
The patient fentanyl concentrations were analyzed using Monolix software version 2018R1 (Lixoft, Orsay, France). Details of the methodology and equations describing this population PK analysis have been described previously.15 In brief, a population nonlinear mixed effect modeling approach was used to generate PK parameter estimates for the population and individual subjects and determine between-subject variability for the parameters. Structural base models included one-, two-, and three-compartment models, with zero-order input, where a single compartment represents the central blood space and the second and third compartments represent peripheral tissue spaces. For each of the structural compartmental models a constant, proportional, and combined error models were evaluated. Patient data, weight, sex, age, ethnicity, and percent TBSA burned were used for covariate analysis. Covariates were incorporated in the final model if there was a significant decrease in the objective function, using stepwise forward addition (P < .05) followed by stepwise backward elimination (P < .01).
The goodness-of-fit of the model was determined by assessing the graphical outputs and the primary diagnostic parameters, including numerical assessment of the −2*log likelihood, Akaike Information Criteria, and Bayesian Information Criterion to determine final model selection. The graphs generated for model evaluation included the estimated population and individual predicted concentrations over time and the observed data versus the population and individual predictions. Additionally, the population and individual weighted residuals versus time, population, and individual weighted residuals versus predictions and the prediction corrected Visual Predictive Check were assessed.
LC-MS/MS Quantification of Blood Fentanyl and Norfentanyl
A liquid chromatography tandem mass spectrometry (LC–MS/MS) method was developed and validated for simultaneous determination of fentanyl and norfentanyl in human plasma. All analyses were performed on a Shimadzu Prominence Ultra-Fast system consisting of binary pumps (LC-20AD), a degassing unit (LC-20A 3R), an auto-sampler (SIL-20AC HT), and a column oven (CTO-20AC) (Shimadzu, Kyoto, Japan) interfaced with an API 4000 tandem mass spectrometer (AB SCIEX, Framingham, MA). The ion source was operated in positive mode, and the optimized mass interface parameters were as follows: curtain gas, 20 psi; gas 1 (nebulizer gas), 55 psi; gas 2 (auxiliary gas), 55 psi; ion spray voltage, 1400 V; and temperature, 600°C. The optimized multiple reaction monitoring (MRM) transitions were 337.2 → 188.1 for fentanyl, 342.2 → 188.1 for stable isotope fentanyl as internal standard, 233.2 → 84.1 for norfentanyl, and 238.2 → 84.1 for stable isotope norfentanyl as internal standard. Chromatographic separation was achieved on Agilent Eclipse Plus C18 column (2.1 × 50 mm, 3.5 μm, Agilent, USA) at the flow rate of 0.8 mL/min, and the column temperature was maintained at 40°C. The mobile phases consisted of 5 mM ammonium formate and 0.02% formic acid in 5% water and 95% acetonitrile as mobile phase A, and 5 mM ammonium formate and 0.02% formic acid in water as mobile phase B. The injection volume was 5.0 μL, and a gradient elution was used for separation with a total run time of 6 min per injection: 0–0.5 minutes, 95% B; 0.5–2.0 minutes, 95%–0% B; 2.0–3.0 minutes, 0% B; 3.0–3.1 minutes, 0%–95% B; 3.1–6.0 minutes, 95% B. Data acquisition and analysis were performed with Analyst 1.6.3 (AB SCIEX, Framingham, MA).
Serum samples (100 μL) were mixed with 300 μL acetonitrile consisting of stable deuterated isotope fentanyl and norfentanyl as internal standard, vortexed, and centrifuged, and then the supernatant was used for LC–MS/MS analysis. The validation of method for determination of fentanyl and norfentanyl in human plasma was carried out according to FDA guidelines for bio-analytical method validation, including specificity, linearity, accuracy and precision, recovery, matrix effect, and stability. The analytical measurement range were 0.02–20 and 0.05–50 ng/ml for fentanyl and norfentanyl, respectively. The intraday and interday precisions (relative standard deviation, RSD, %) were all within 11.5% for both fentanyl and norfentanyl, and the accuracy (relative error, RE, %) was less than 7.3%. The recoveries were over 91.0% for both analytes and there was no obvious matrix effect. The limit of quantification for fentanyl and norfentanyl were 0.02 and 0.05 ng/ml, respectively. The results of short-term stability (room temperature for 4 hours), three freeze-thaw stability, and auto-sampler stability (processed samples at 15°C for 4 hours in autosampler) were found to be within the assay variability limits during the entire process.
RESULTS
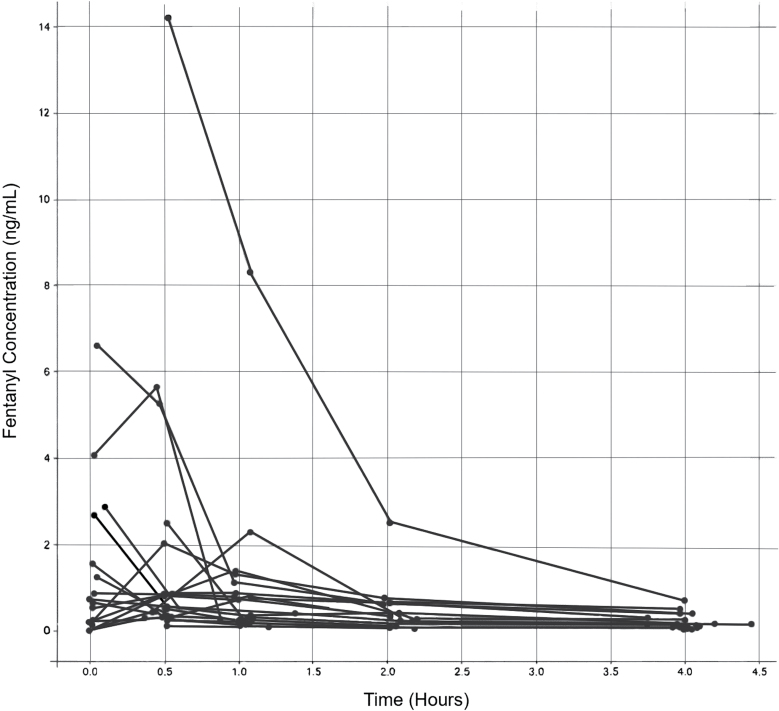
A total of 14 patients (10 males and 4 females) were included in the PK analysis. Patient demographics are summarized in Table 1. A total of 99 data points were used for population analysis. The average total dose of fentanyl was 2.25 µg/kg (SD 1.80 µg/kg). Patients were administered either a single intravenous bolus or the bolus split between 2 and 3 smaller boluses, typically administered 10–15 minutes apart. Each individuals’ specific dosing was used in the PK model as either a single doing event or multidosing events. The patient concentrations over time are displayed in Figure 1. The mean (SD) maximum fentanyl concentration was 2.93 (4.20) ng/ml. Norfentanyl concentrations were below the assay’s limit of quantification.
Table 1.
Demographics of pediatric burn patients
| Characteristic | Mean value (SD)* | Median | Q1, Q3 |
|---|---|---|---|
| Patients (no.) | 14 | ||
| Gender (no.) | |||
| Males | 10 | ||
| Females | 4 | ||
| Ethnicity (no.) | |||
| Hispanic/Latino | 11 | ||
| Caucasian | 1 | ||
| African American | 1 | ||
| Unknown | 1 | ||
| Burn type (no.) | |||
| Flame | 9 | ||
| Electrical | 1 | ||
| Scald | 4 | ||
| Age (yr) | 9.6 (5.5) | 10.5 | 5, 14 |
| Weight (kg) | 43.8 (24.2) | 37.4 | 24.4, 63.6 |
| TBSA % | 24.3 (11.6) | 21.5 | 15.5, 28.0 |
| Mechanical ventilation (no.) | |||
| Yes | 5 | ||
| No | 9 | ||
| Total length of stay | 60.7 (40.2) | 21.5 | 15.5, 28.0 |
SD, Standard deviation; Q1, 1st Quartile; Q3, 3rd Quartile; no., total number; TBSA %, percent of total body surface area burned.
*Values are Mean (SD) except where indicated as total number (no.)
Figure 1.
Pediatric burn patient measured serum fentanyl concentrations (ng/ml) over time (hr).
The PK profiles were biexponential in nature. A two-compartmental model with a proportional error model was the best operative for describing the time course of the drug profiles for fentanyl in these pediatric burn patients. The compartmental model was parameterized using clearance (CL), inter-compartmental clearance (Q), and Vd for the central (V1) and peripheral compartment (V2). Weight was the only covariate determined to significantly affect the fentanyl PK and was included in the final model. The equations used to calculate CL and Vd, with weight as a covariate, are presented in equations 5 and 6, and are described in further detail in the Monolix user’s guide,
| (5) |
| (6) |
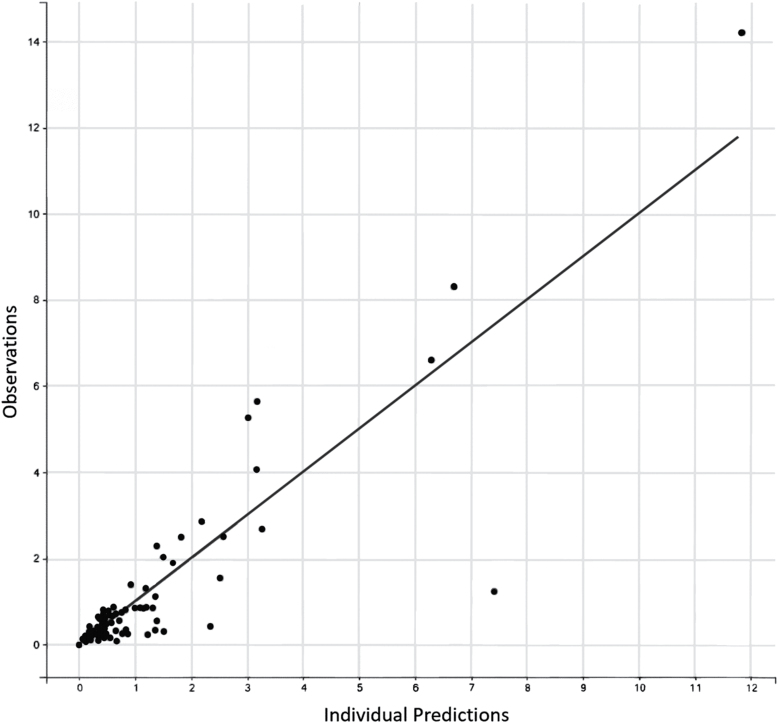
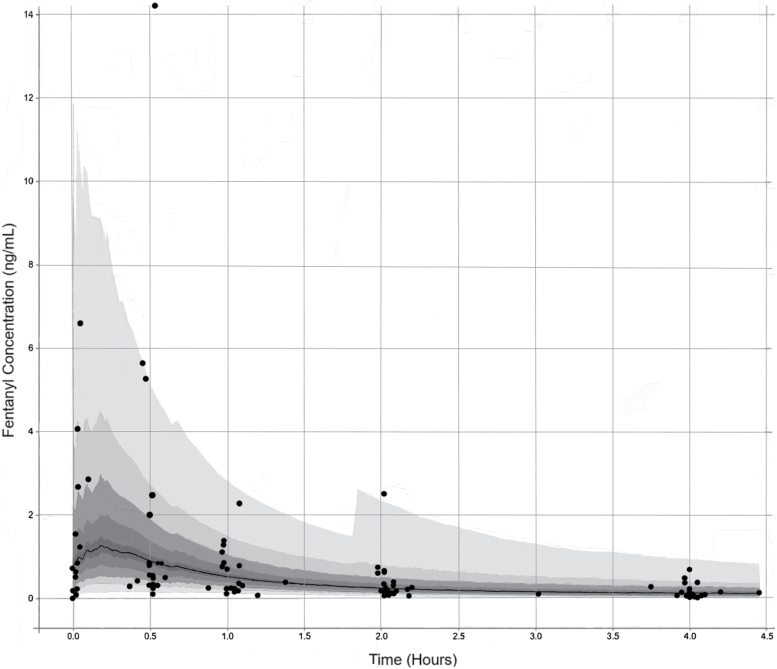
The observed and fitted PK profiles are presented in Figure 2. The Visual Predictions are presented in Figure 3 with the observed data overlaid. The population clearance and intercompartmental clearance were 7.19 and 2.16 L/hour, respectively, and the volume of distribution for the central and peripheral compartments were 4.01 and 25.1 L, respectively. Individual patient clearances and volumes of distribution had extensive variability as summarized in Table 2. The three patients that were 3 years old or less did have the smallest volumes of distributions ranging from 3.02 to 4.98 L. These patients’ clearances trended towards the lower range (3.70–5.27 L/hour), but the values do overlap with the older patients.
Figure 2.
A plot of the measured concentrations of fentanyl against the population pharmacokinetic model predicted fentanyl concentrations demonstrating the model has good predictive outcomes compared with the patient data.
Figure 3.
A Visual Predictive plot with the dark grey dots representing the patient observed data, the black line depicting the model predicted median, and the grey zones each representing the percentiles divided into 10ths. The observed patient data demonstrate the broad interpatient variability from the median.
Table 2.
Fentanyl pharmacokinetic parameter estimates in pediatric burn patients
| Population parameter estimations | |||||
|---|---|---|---|---|---|
| Parameter | Values | SE | rSE (%) | ||
| CL (L/hr) | 7.19 | 4.5 | 62.5 | ||
| Q (L/hr) | 2.16 | 1.58 | 73 | ||
| V1(L) | 4.01 | 2.72 | 67.7 | ||
| V2 (L) | 25.1 | 16.6 | 66.2 | ||
| Individual parameter estimations by conditional mean | |||||
| Parameter | Minimum | Q1 | Median | Q3 | Maximum |
| CL (L/hr) | 3.59 | 6.1 | 12 | 19.3 | 73.4 |
| V1 (L) | 2.93 | 6.39 | 27.1 | 64.9 | 428 |
| Q (L/hr) | 2.27 | 7.2 | 18.4 | 55.7 | 104 |
| V2 (L) | 26.2 | 75.3 | 104 | 412 | 825 |
SE, Standard Error; rSE, residual Standard Error; CL, Clearance; Q, intercompartmental clearance; V1, volume of distribution of the central compartment; V2, volume of distribution of the peripheral compartment; Q1, first quartile; Q3, third quartile.
Review of patient records to identify drugs that may significantly affect fentanyl PK during the timeframe the samples were collected did not identify any drugs that are reported in the literature to be moderate to strong inducers or inhibitors of the primary opioid metabolizing enzymes.
DISCUSSION
The present study is the first to report the population PK of fentanyl in pediatric burn patients and characterizes the broad interpatient variability. These data further support the published literature characterizing the extensive variability observed between individual fentanyl concentration profiles and PK estimates. The population clearances and volume of distributions determined here are similar to the values reported in pediatric cardiac patients8 and other fentanyl PK studies in pediatric populations.16,17 Comparing this study’s full range of variability to published literature is not possible as these publications only report standard deviation or error values and do not fully disclose the full range of variability by reporting quartile ranges, minimum and maximum values. Weight was the only covariate that significantly decreased the objective function of the model, which is also similar to previously reported literature.8 Although other covariates may have an impact on fentanyl PK in pediatric burn patients, the present study only evaluated 14 patients across a range of ages, burn sizes, and other characteristics, thus making it difficult to have sufficient power in this heterogenous population to definitively define dosing regimens. Moreover, due to the small sample size the current study did not incorporate important burn-related changes that could be impactful on physiological pathways such as TBSA and duration of time since initial burn injury, which the authors do plan to investigate further investigations.
Another variable which affects the ability to accurately model this diverse patient population is the ability to accurately capture the individual patient doses and the exact time they were delivered. Patients are commonly delivered fentanyl in a number of small doses or micro-doses titrated to effect, which may not be accurately captured in the electronic medical records. Although one can try to predict when these doses may have been administered by examining the peaks in the fentanyl concentration profile, these would be assumptions within the model and potentially contribute to some inaccuracies. The authors did encounter these issues with some of the patients and did need to adjust the model dosing input to reflect the approximate time subsequent doses were administered. Despite the small sample size, this study provides valuable PK data to aid clinicians in further optimizing fentanyl dosing regimens in pediatric burn patients.
Studies have focused identifying the most significant factors contributing to variability in fentanyl PK.7,11 The most significant factors include 1) drugs that can induce or inhibit the CYP3A4 pathway, 2) liver function, and 3) age (particularly the elderly), whereas patient body mass index and gender were questionable likely due to large heterogeneity in the published literature.7 Although the authors focused this review with an emphasis on transdermal fentanyl for cancer patients, there are a number of factors that are applicable to all populations and some more specifically for burn patients.
An impactful factor influencing opioid PK is drug competition for common metabolic pathways. Although traditional teaching is that fentanyl is metabolized by the CYP3A4 pathway,18 recent studies suggest that other pathways may also play significant roles.7,13,19 Patients in the present study received a number of other drugs (eg, hydrocodone, hydromorphone, methadone, and midazolam) that serve as substrates and compete for the same metabolizing pathways (eg, CYP3A4 and CYP2D6). These types of competitive drug interactions may have some impact on the fentanyl metabolism and be a contributing factor to intra- and interpatient variability, while not being significant enough to result in severe inhibition leading to significant increases in fentanyl concentration and adverse effects. Additionally, burn patients may receive antifungals during their treatment. Antifungals (eg, itraconazole, ketoconazole, and voriconazole) induce small changes in fentanyl concentration.7,13 Patients in the present study did not receive antifungals at the time the samples were collected. These patients were only on the study for the first 2 weeks of their stay and did not acquire any fungal infections.
Burn patients experience severe alterations in their albumin levels which may potentially affect fentanyl PK. A significant reduction in albumin due to high vascular permeability and loss of important proteins through burn wounds is common in patients with severe burns.20 Fentanyl binds to plasma proteins such as albumin and alpha-1-acid glycoprotein. Studies of the impact of hypoalbuminemia on fentanyl PK have discrepant results, with one study reporting that fentanyl concentrations were significantly reduced in patients with low albumin compared with normal albumin,21 whereas others have reported no clinically relevant influence.7,22 Several patients in the present study did warrant albumin administration on the days of sample collection. The impact of hypoalbuminemia and albumin treatment was not evaluated in the present study and warrants further investigation, specifically evaluating the association between plasma proteins and free unbound fentanyl, as this is likely to have a significant impact on fentanyl PK in burn patients.
Additional factors that may affect fentanyl metabolism include organ function, specifically liver and cardiac function. Reduced liver function cannot only decrease the function of the drug metabolizing pathways but also plasma protein production such as albumin. It has been demonstrated that patients with nonalcoholic liver disease have decreased CYP3A function.23 Reduced cardiac output may result in decrease drug distribution throughout the body and through the liver for metabolism, resulting in a decreased clearance. Alternatively, increased cardiac output, as often observed during the hypermetabolic state following severe burn injury, can result in an increased clearance as a result of increased blood flow through the liver.12 Genetic polymorphisms, particularly in cytochrome P450 enzymes, are of growing interest. Although a plethora of studies are elucidating the impact of clinically significant variants that have been identified to impact opioid metabolism,19,24–27 further research needs to be conducted to provide stronger evidence of clinically significant variants associated with alterations in specifically fentanyl PK.
CONCLUSIONS
Pediatric burn patients have extensive variability in fentanyl PK due to burn-related pathophysiology as well as patient characteristics and other drug administration regimens. Development of a comprehensive pediatric opioid dosing regimen will require elucidation of the impact of age, metabolic rate, concurrent drug administration, and effects of open wounds on drug pharmacokinetics. This study emphasizes the need for future investigations and complex analysis focusing on a larger number of patients, comparison controls, as well as incorporating additional patient information including but not limited to paired albumin data, hemodynamic/cardiac parameters, and identification of genetic polymorphisms of clinical relevance to opioid metabolism.
REFERENCES
- 1. Prakash S, Fatima T, Pawar M. Patient-controlled analgesia with fentanyl for burn dressing changes. Anesth Analg 2004;99:552–5, table of contents. [DOI] [PubMed] [Google Scholar]
- 2. de Castro RJ, Leal PC, Sakata RK. Pain management in burn patients. Braz J Anesthesiol 2013;63:149–53. [DOI] [PubMed] [Google Scholar]
- 3. Anand KJ, Willson DF, Berger J, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010;125:e1208–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peng PWH, Sandler AN.. Anesthesiology. Vol. 90 American Society of Anesthesiologists. Howell B, Glass S, PSA, et al. , editors. American Society of Anesthesiologists, etc.; 1999, accessed 13 Jan. 2019; available from http://anesthesiology.pubs.asahq.org/article.aspx?articleid=1946720 [Google Scholar]
- 5. Choi L, Ferrell BA, Vasilevskis EE, et al. . Population pharmacokinetics of fentanyl in the critically ill. Crit Care Med 2016;44:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bentley JB, Borel JD, Nenad RE Jr, Gillespie TJ. Age and fentanyl pharmacokinetics. Anesth Analg 1982;61:968–71. [PubMed] [Google Scholar]
- 7. Evelien Kuip CJ, M Kuip EJ, Zandvliet ML, W Koolen SL, J Mathijssen RH, D van der Rijt CC. REVIEW A review of factors explaining variability in fentanyl pharmacokinetics; focus on implications for cancer patients. Br J Clin Pharmacol Br J Clin Pharmacol 2017;83:294–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Driest SL, Marshall MD, Hachey B, et al. . PAEDIATRIC CLINICAL PHARMACOLOGY pragmatic pharmacology: population pharmacokinetic analysis of fentanyl using remnant samples from children after cardiac surgery. Br J Clin Pharmacol 2016;81:1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ginsberg G, Hattis D, Sonawane B, et al. . Evaluation of child/adult pharmacokinetic differences from a database derived from the therapeutic drug literature. Toxicol Sci 2002;66:185–200. [DOI] [PubMed] [Google Scholar]
- 10. Koehntop DE, Rodman JH, Brundage DM, Hegland MG, Buckley JJ. Pharmacokinetics of fentanyl in neonates. Anesth Analg. 1986;65:227–32PMID:3954090. [PubMed] [Google Scholar]
- 11. Reilly CS, Wood AJ, Wood M. Variability of fentanyl pharmacokinetics in man. Computer predicted plasma concentrations for three intravenous dosage regimens. Anaesthesia 1985;40:837–43. [DOI] [PubMed] [Google Scholar]
- 12. Han T, Harmatz JS, Greenblatt DJ, Martyn JA. Fentanyl clearance and volume of distribution are increased in patients with major burns. J Clin Pharmacol 2007;47:674–80. [DOI] [PubMed] [Google Scholar]
- 13. Ziesenitz VC, König SK, Mahlke NS, Skopp G, Haefeli WE, Mikus G. Pharmacokinetic interaction of intravenous fentanyl with ketoconazole. J Clin Pharmacol 2015;55:708–17. [DOI] [PubMed] [Google Scholar]
- 14. Godwin Z, Lima K, Greenhalgh D, Palmieri T, Sen S, Tran NK. A retrospective analysis of clinical laboratory interferences caused by frequently administered medications in burn patients. J Burn Care Res 2016;37:e10–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grimsrud KN, Ivanova X, Sherwin CM, Palmieri TL, Tran NK. Identification of cytochrome P450 polymorphisms in burn patients and impact on fentanyl pharmacokinetics: a pilot study. J Burn Care Res 2019;40:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koren G, Goresky G, Crean P, Klein J, MacLeod SM. Pediatric fentanyl dosing based on pharmacokinetics during cardiac surgery. Anesth Analg 1984;63:577–82. [PubMed] [Google Scholar]
- 17. Ginsberg B, Howell S, Glass PS, et al. . Pharmacokinetic model-driven infusion of fentanyl in children. Anesthesiology 1996;85:1268–75. [DOI] [PubMed] [Google Scholar]
- 18. Smith HS. Opioid metabolism. Mayo Clin Proc 2009;84:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grimsrud KN, Ivanova X, Sherwin CM, Palmieri TL, Tran NK. Identification of cytochrome P450 polymorphisms in burn patients and impact on fentanyl pharmacokinetics: a pilot study. J Burn Care Res 2018;40:91–6. doi:10.1093/jbcr/iry053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pérez-Guisado J, de Haro-Padilla JM, Rioja LF, Derosier LC, de la Torre JI. Serum albumin levels in burn people are associated to the total body surface burned and the length of hospital stay but not to the initiation of the oral/enteral nutrition. Int J Burns Trauma 2013;3:159–63. [PMC free article] [PubMed] [Google Scholar]
- 21. Nomura M, Inoue K, Matsushita S, et al. . Serum concentration of fentanyl during conversion from intravenous to transdermal administration to patients with chronic cancer pain. Clin J Pain 2013;29:487–91. [DOI] [PubMed] [Google Scholar]
- 22. Barrett JS, Della Casa Alberighi O, Läer S, Meibohm B. Physiologically based pharmacokinetic (PBPK) modeling in children. Clin Pharmacol Ther 2012;92:40–9. [DOI] [PubMed] [Google Scholar]
- 23. Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A activity and expression in nonalcoholic fatty liver disease. Drug Metab Dispos 2015;43:1484–90. [DOI] [PubMed] [Google Scholar]
- 24. Zhang W, Chang YZ, Kan QC, et al. . CYP3A4*1G genetic polymorphism influences CYP3A activity and response to fentanyl in Chinese gynecologic patients. Eur J Clin Pharmacol 2010;66:61–6. [DOI] [PubMed] [Google Scholar]
- 25. Richards-Waugh LL, Primerano DA, Dementieva Y, Kraner JC, Rankin GO. Fatal methadone toxicity: potential role of CYP3A4 genetic polymorphism. J Anal Toxicol 2014;38:541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li HB, Lin R, Zhou XJ, Li W, Liu ZQ, Zhu LP. Effects of OPRM1, ABCB1 and CYP2D6 single nucleotide polymorphisms on clinical efficacy of sufentanil-propofol anesthesia in patients undergoing gynecologic laparoscopic surgery: a preliminary study. Int J Clin Exp Med 2016;9:23048–59. [Google Scholar]
- 27. Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 2009;41:89–295. [DOI] [PubMed] [Google Scholar]