Figure 6.
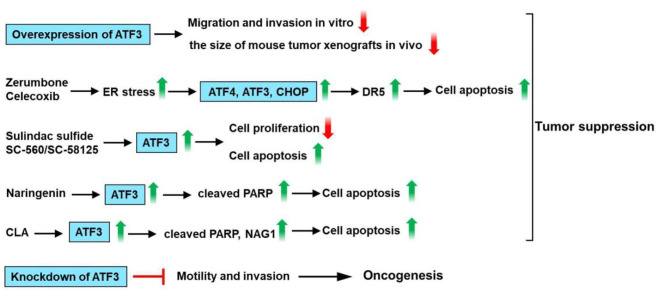
ATF3 plays a critical role in colon cancer. ATF3 overexpression could inhibit the migration and invasion of the HCT116 cells and decrease the size of mouse tumor xenografts. ATF3 promotes death receptor 5 (DR5) induction and apoptotic cell death upon zerumbone (a bioactive sesquiterpene) or celecoxib (a selective inhibitor of cyclooxygenase 2) treatment in human p53-deficient colorectal cancer cells. ATF3 is upregulated by treatment with cyclooxygenase (Cox) inhibitor (sulindac sulfide) or Cox-1 specific inhibitor SC-560 or Cox-2 specific inhibitor SC-58125, suggesting that ATF3 may be involved in sulindac sulfide/SC-560/SC-58125-induced apoptosis and anti-tumorigenic effect in human colon cancer cells. Naringenin (NAR) upregulates ATF3 expression in colon cancer cells. Furthermore, ATF3 overexpression enhances NAR-modulated cleavage of PARP. Trans-10, cis-12-conjugated linoleic acid (CLA) could increase ATF3 expression. In addition, ATF3 modulates trans-10, cis-12-CLA-stimulated non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) expression and apoptosis in colon cancer cells. These findings suggest that ATF3 acts as a tumor suppressor for colon cancer. However, siRNA-mediated knockdown of ATF3 attenuates motility and invasion of the colon cancer cell. These observations suggest that ATF3 plays a dual role in colon cancer.