Abstract
Background
Central nervous system levels of tumor necrosis factor‐alpha (TNF‐α), a pro-inflammatory cytokine, regulate the neuroinflammatory response and may play a role in age-related neurodegenerative diseases. The longitudinal relation between peripheral levels of TNF-α and typical brain aging is understudied. We hypothesized that within-person increases in systemic TNF‐α would track with poorer brain health outcomes in functionally normal adults.
Methods
Plasma-based TNF‐α concentrations (pg/mL; fasting morning draws) and magnetic resonance imaging were acquired in 424 functionally intact adults (mean age = 71) followed annually for up to 8.4 years (mean follow-up = 2.2 years). Brain outcomes included total gray matter volume and white matter hyperintensities. Cognitive outcomes included composites of memory, executive functioning, and processing speed, as well as Mini-Mental State Examination total scores. Longitudinal mixed-effects models were used, controlling for age, sex, education, and total intracranial volume, as appropriate.
Results
TNF‐α concentrations significantly increased over time (p < .001). Linear increases in within-person TNF‐α were longitudinally associated with declines in gray matter volume (p < .001) and increases in white matter hyperintensities (p = .003). Exploratory analyses suggested that the relation between TNF‐α and gray matter volume was curvilinear (TNF‐α 2p = .002), such that initial increases in inflammation were associated with more precipitous atrophy. There was a negative linear relationship of within-person changes in TNF‐α to Mini-Mental State Examination scores over time (p = .036) but not the cognitive composites (all ps >.05).
Conclusion
Systemic inflammation, as indexed by plasma TNF‐α, holds potential as a biomarker for age-related declines in brain health.
Keywords: Inflammation, Brain aging, Cognition, Gray matter volume, Neuroimaging
In old age, there appears to be a chronic, low-grade, neuroinflammatory response within the central nervous system (1). This phenomenon, coined “inflamm-aging,” is related to an increased ratio of pro-inflammatory relative to anti-inflammatory cytokines (2). Various mechanisms have been proposed to explain inflamm-aging, such as age-related free radical damage to DNA involved in cytokine regulation, leakage of harmful gut microbiota into the circulatory system, mitochondrial dysfunction, cellular senescence, and priming of microglia (3). Prolonged pro-inflammatory cytokine activity within the brain may exert negative effects on cellular functions, including synaptogenesis, long-term potentiation, and dendritic structuring (4). Neuroinflammation also suppresses neurotrophins, which are critical for neuronal function and survival (5). On a behavioral level, chronic inflammation is posited to contribute to cognitive impairments, neuropsychiatric symptoms, and an exaggerated “sickness” response (eg, reduced appetite, lethargy, and social withdrawal) (4,6,7). Higher levels of inflammation have additionally been associated with declines in mobility and other aspects of physical disability in older adults (8).
Tumor necrosis factor‐alpha (TNF‐α) is a pro-inflammatory cytokine primarily produced by monocytes and macrophages (9). It is considered a “master regulator” of the innate immune response based on studies demonstrating that TNF‐α blockade directly influences production of a variety of other pro- and anti-inflammatory factors, such as interleukin (IL)-1, IL-6, IL-8, GM-CSF, and adiponectin (9). TNF‐α inhibitors are U.S. Food and Drug Administration (FDA) approved for the treatment of rheumatoid arthritis, psoriasis, and other autoimmune and inflammatory conditions. Accumulating evidence suggests a possible role of TNF‐α in age-related neurodegenerative diseases, such as Alzheimer's disease. For example, cerebrospinal fluid levels of TNF‐α are increased 25-fold in Alzheimer's disease patients relative to healthy controls and correlate with clinical decline (10,11). Overrepresentation of certain single-nucleotide polymorphisms within the promoter region of the TNF-α gene increase risk for Alzheimer's disease (12). A recent systematic review of the literature, based primarily on animal model research, suggested beneficial effects of TNF‐α inhibitors in Alzheimer's disease on behavioral outcomes and underlying pathology (13).
The role of TNF‐α in typically aging adults (ie, individuals who do not show evidence of cognitive or functional decline suggestive of underlying neurodegenerative disease or other neurological condition), particularly when measured in the periphery, is poorly understood. Peripheral blood-based measurements of inflammation hold several advantages as potential biomarkers of unhealthy brain aging, including their minimally invasive nature and low cost. They also signal across the blood–brain barrier and correlate with inflammatory status in the central nervous system (14,15). A small number of studies have evaluated the relation of systemic TNF‐α, measured via peripheral blood draw, to neuroimaging and cognitive outcomes in older adults with somewhat mixed findings (16). In cross-sectional analyses, Zhang and colleagues (17) found TNF‐α to negatively correlate with gray matter volume (GMV) in a large sample of older adults. By contrast, Papenberg and colleagues (18) did not find any correlation between TNF‐α and GMV in a 6-year longitudinal study. In a relatively small sample of older adults with major depressive disorder (N = 31), Smagula and colleagues (19) found a cross-sectional relation between TNF‐α and white matter hyperintensities (WMH) but not GMV or executive functioning. The observed discrepancies in findings across studies may be explained by methodological differences in study design (eg, cross-sectional vs longitudinal) and cytokine platform measurement techniques. In addition, the aforementioned studies did not consistently exclude individuals with the pre-dementia syndrome mild cognitive impairment, which may have influenced the observed results (20).
The primary aim of this study was to clarify the longitudinal relation between systemic TNF‐α trajectories and brain health in functionally normal, typically aging older adults. On the basis of the broader literature on inflamm-aging (1), peripheral levels of TNF‐α in plasma were expected to increase with age over time. In turn, within-person changes in plasma TNF‐α were hypothesized to negatively relate to GMV and positively relate to WMH burden over time. The longitudinal relation between systemic TNF‐α and cognition was additionally investigated, both globally and in specific cognitive domains shown to decline with age, including executive functioning, memory, and processing speed. Exploratory analyses were conducted to assess for possible curvilinear relationships of plasma TNF‐α to neural and cognitive outcomes.
Methods
Participants
Participants were enrolled in the Hillblom Network for the Prevention of Age-Associated Cognitive Decline at the University of California, San Francisco (UCSF) Memory and Aging Center. The Hillblom cohort includes community-dwelling older adults who have been recruited from the Bay Area since 2000 via community outreach events, flyers, and newspaper advertisements. For the present analyses, participants completed annual study visits, occurring approximately 15–18 months apart. Plasma TNF‐α concentrations, neuroimaging (magnetic resonance imaging [MRI]), and cognitive data were acquired at every study visit (baseline and follow-up visits). Comprehensive neuropsychological and neurological evaluations were also performed during every study visit, including interview of a collateral informant. As part of these evaluations, height and weight were measured for calculation of body mass index and blood pressure was recorded. Fasting blood samples were obtained and sent to UCSF Clinical Laboratories for measurement of Hemoglobin A1c (HbA1c) and high-sensitivity C-reactive protein (hs-CRP) using standard procedures. Inclusion criteria, at every study visit, were the following: (i) clinically normal per consensus conference with a board-certified neuropsychologist and neurologist, (ii) absence of functional decline, per collateral informant report, based on a Clinical Dementia Rating global score of 0 (21), and (iii) no history of a neurological condition (eg, epilepsy, stroke). The present analyses were conducted on a subset (baseline n = 424) of the larger Hillblom Network (total n = 913) with available neuroimaging and plasma (TNF‐α) data. The subset was similar to the larger Hillblom cohort with respect to a number of characteristics, including, sex, and global cognition (Mini-Mental State Examination [MMSE]; all ps >.05), but tended to be older (mean age of 71 compared to 63, p < .001). Because the Hillblom Network is an active and ongoing longitudinal study, the number of study visits for participants included in the present analyses ranged from one (baseline visit only) to a maximum of eight, depending on how long a given participant had been enrolled in the study (mean number of study visits = 2.33). All participants provided written informed consent and the UCSF Committee on Human Research approved the study protocol (no. 10-02076).
Plasma TNF‐α Measurement
The procedure used in this sample to quantify TNF‐α concentrations in plasma has been published and detailed elsewhere (22). Briefly, 12-hour fasting blood draws were acquired in the morning and centrifuged for 15 minutes at 2000g at 4°C. The captured plasma was placed in 500 µL polypropylene cryovials at −80°C until analysis. In preparation for assay initiation, the plasma samples were slowly brought to room temperature. TNF‐α levels were then measured using the Meso Scale Diagnostics, LLC, human pro-inflammatory panel 1 V-PLEX kit (Rockville, MD) according to standard manufacture guidelines. Discovery Workbench v4.0 (software provided by the manufacturer) was used to quantify TNF‐α concentrations according to sample dilution and compared to the supplied in-assay curve. Published lower limit of detectability, lower limit of quantification, and upper limit of quantification (pg/mL) on this platform are 0.01–0.13, 0.69, and 248, respectively. We are unaware of any participants being excluded by the laboratory for falling outside of the detectable range, but this information was not specifically recorded by the laboratory, which reflects a limitation to this study. On the basis of prior work using this same platform in the Hillblom cohort (23), if any participants were excluded by the laboratory, we expect this number to be very small.
Neuroimaging Acquisition and Analysis
Protocol
MRI data were acquired at the UCSF Neuroscience Imaging Center using a Siemens Trio 3T scanner. The protocol includes a T1-weighted structural scan, acquired sagittally, with the following parameters: acquisition time = 8 minutes and 53 seconds; field of view = 160 × 240 × 256 mm with isotropic voxel resolution = 1 mm3; repetition time = 2300 ms; echo time = 2.98 ms; time inversion = 900 ms; flip angle = 9°. Fluid attenuated inversion recovery images were acquired for quantification of WMH (slice thickness = 1.00 mm; slices per slab = 160; in-plane resolution = 0.98 × 0.98 mm; matrix = 256 × 256; repetition time = 6000 ms; echo time = 388 ms; time inversion = 2100ms; flip angle = 120°).
T1 processing
T1-weighted images were visually inspected for quality prior to processing; images containing excessive artifact or motion were excluded. The N3 algorithm was used to adjust for magnetic field bias (24). Tissue segmentation was achieved via SPM12's unified segmentation procedure (25). A study-specific template was created with Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) for warping of individual participant T1-weighted images (26). Nonlinear and rigid-body registration was implemented to normalize and modulate the images within the study-specific template space. An 8-mm full width half maximum Gaussian kernel was used for smoothing. Linear and nonlinear transformations between DARTEL's space and International Consortium of Brain Mapping (ICBM) space were performed to enable registration with a brain parcellation atlas. Volumetric quantification entailed transforming a standard parcellation atlas (27) into ICBM space and summing all gray matter within each parcellated region. Total intracranial volume (TIV) and total GMV (in L) were calculated in Montreal Neurological Institute (MNI) space.
WMH quantification
WMHs were calculated (in mm3) using the fluid attenuated inversion recovery and T1-weighted images. After raw scans were visually inspected for quality, a fully automated segmentation process was implemented, which is based on a regression algorithm and uses Hidden Markov Random Field with Expectation Maximization Software (28,29). Resultant segmentations were all assessed manually for accuracy.
Cognitive Outcomes
The MMSE was administered as a screener of global cognitive status (30). Scores range from 0 to 30, with higher scores indicating better cognition. In addition, performance in specific cognitive domains was measured using composite measures of episodic memory, executive functioning, and processing speed, as described previously (31,32). For each composite, sample-based z scores were calculated for individual subtests within the composite and then these subtest z scores were averaged to create the corresponding composite. The episodic memory composite included visual memory on the Benson Figure Recall (33) and verbal memory on the California Verbal Learning Test, second edition (34), including the immediate recall total, delayed free recall total, and recognition discriminability (d′). The executive functions composite (32) was derived from Stroop interference, modified trail making test, digit span backward, phonemic fluency (number of D-words per minute), and design fluency (Condition 1, Delis–Kaplan Executive Function System). The processing speed composite included six visuospatial processing speed tests, normalized relative to a healthy young adult comparison group, as detailed by Kerchner and colleagues (31) (the mental rotation task was excluded in the present analyses because its use was discontinued in the Hillblom Network). Greater scores correspond to better performance for the episodic memory and executive functioning composite scores. By contrast, lower scores indicate better performance (ie, faster reaction times) on the processing speed composite.
Statistical Analyses
Longitudinal changes in TNF‐α levels were assessed by including TNF‐α as the dependent variable in a linear mixed-effect model with time (in years) as the predictor variable. Mixed-effects models were also used to evaluate the longitudinal relationship of TNF‐α to brain structural (GMV and WMH) and cognitive (MMSE, episodic memory, executive functioning, and processing speed) outcomes of interest. When testing for possible curvilinear effects, a quadratic term was included in the model in addition to the linear term to assess for a quadratic fit above and beyond any linear relationship. Baseline age, sex, and educational attainment (in years) were entered as covariates in all of the models containing cognitive outcomes. Baseline age, sex, and TIV at baseline were included as covariates when evaluating relations to GMV and WMH. To clarify whether interindividual (between-subject) differences and/or intraindividual (within-subject) changes in TNF‐α were associated with our outcome variables, TNF‐α was decomposed into between-person and within-person variance in our models, as described previously (35). All longitudinal analyses were conducted in Stata 15.1 and modeled with random intercepts and slopes. When models failed to converge, random intercept only models were run. To meet assumptions of normality, WMH, TNF‐α, and MMSE scores were logarithmically transformed when treated as dependent variables in analyses.
Post Hoc Analysis of Brain Regional Effects
In addition to evaluating the relationship between TNF‐α and total GMV, post hoc analyses were conducted to investigate potential relations of TNF‐α to GMV changes within functional brain networks that have been established in the neuroscience literature (36). In this way, possible effects of TNF‐α on GMV within specific brain regions could be explored. The procedure for constructing our volumetric gray matter networks is published and detailed elsewhere (32), and thus will only be summarized here. As described previously (32), we defined several brain networks using a set of 75 healthy older participants from the Hillblom cohort (mean age = 65.3 ± 10 years, 33 females/42 males, mean education = 17.3 ± 2.1 years, 68 right handed/7 left handed), 58 of whom were also included in primary analyses for this study. All participants used for network construction underwent a T1-weighted structural MRI scan, which was acquired and processed using the same pipeline as described earlier for this study. They additionally received a T2*-weighted echoplanar task-free (resting state) functional MRI (tf-fMRI) scan with the following parameters: acquisition time = 8 minutes and 6 seconds; axial orientation with interleaved ordering; field of view = 230 × 230 × 129 mm; matrix size = 92 × 92; effective voxel resolution = 2.5 × 2.5 × 3.0 mm; repetition time = 2000 ms; echo time = 27 ms; 240 volumes total. The tf-fMRI data were preprocessed in SPM12 using a standard pipeline that is detailed elsewhere (32), which includes slice-time correction, motion correction, coregistration to the T1-weighted structural scan, spatial smoothing, and normalization to MNI space. The whole-brain functional connectome was then determined using 228 regions from the Brainnetome atlas (37). Briefly, we used a modularity-based method for identification of nodes that comprised each module or “intrinsic connectivity network” of interest, using a similar strategy to that used by Power and colleagues (38), implementing the Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/). Networks used for this study included the default mode network, frontoparietal/executive network, and dorsal attention network, as well as a hippocampal network and a subcortical network. The Brainnetome regions comprising the tf-fMRI networks were then applied to every participant's T1-weighted structural scan, at each study visit, in order to extract network-level GMVs. More specifically, the volumes of all brain regions forming a given network were added together to calculate each network's overall GMV. The specific regions of interest included within each GMV network are provided in Supplementary Table 1.
To evaluate potential longitudinal relationships between TNF‐α levels and volumetric changes within the aforementioned brain networks, the network-level GMVs were entered as dependent variables in mixed-effects models, as described in detail earlier (see Statistical Analyses section), controlling for baseline age, sex, and baseline TIV. We additionally investigated the longitudinal relationship between TNF‐α and GMV changes within the bilateral hippocampi specifically, given that the hippocampi are implicated in early stages of age-related neurodegenerative conditions such as Alzheimer's disease.
Results
Cross-Sectional Analyses
Descriptive statistics for the sample at baseline (n = 424) are presented in Table 1. Raw scores of individual subtests within each cognitive composite are provided in Supplementary Table 2. Approximately 23% of the samples were carriers of at least one apolipoprotein ε4 allele. TNF‐α levels were similar between ε4 carriers and noncarriers (p = .55). In cross-sectional analyses (at baseline), age demonstrated a strong, positive relation with TNF‐α, which remained significant after adjusting for sex and education (β = 0.33, p < .001, n = 421). TNF‐α was negatively and significantly associated with total GMV upon correcting for TIV, age, and sex (β = −0.11, p = .02, n = 228). The addition of a quadratic term did not significantly contribute to the model in predicting GMV (∆R2 = .001, ∆F = .39, p = .53). The relation between TNF‐α and WMH controlling for TIV, age, and sex was not statistically significant (β = −0.02, p = .81, n = 132). The addition of a quadratic term did not significantly contribute to the model in predicting WMH (∆R2 ≤ .001, ∆F = .03, p = .87).
Table 1.
Descriptive Statistics of the Study Sample at Baseline
| All Participants (N = 424) | Participants With 1 Visit (N = 174) | Participants With 2 Visits (N = 84) | Participants With 3–8 Visits (N = 166) | |
|---|---|---|---|---|
| Age (years) | 71.35 (8.08) | 71.98 (8.57) | 71.40 (7.98) | 70.67 (7.56) |
| Education (years) | 17.48 (2.03)* | 17.20 (2.01) | 17.49 (2.05) | 17.77 (2.00) |
| Sex (% female) | 57.30% | 63.20% | 58.30% | 50.60% |
| TNF‐α (pg/mL) | 2.61 (1.37) | 2.73 (1.52) | 2.61 (0.98) | 2.47 (1.38) |
| APOE (% ε4 carrier) | 22.50%† | 24.40% | 25.00% | 19.30% |
| MMSE (total score) | 29.16 (1.14)‡ | 29.01 (1.36) | 29.15 (0.99) | 29.34 (0.91) |
| SBP (mm Hg) | 133.83 (16.85)§ | 133.69 (14.32) | 135.28 (18.94) | 133.28 (18.28) |
| Hemoglobin A1c | 5.52 (0.37)|| | 5.50 (0.39) | 5.58 (0.35) | 5.51 (0.35) |
| BMI (kg/m2) | 25.82 (5.80)¶ | 26.29 (7.72) | 25.60 (4.53) | 25.45 (3.64) |
| Current smoker (%) | 2.48%# | 2.97% | 0.00% | 3.18% |
| Total GMV (L) | 0.61 (0.06)** | 0.60 (0.05) | 0.60 (0.06) | 0.61 (0.06) |
| WMH (mm3) | 2259 (3014)†† | 3085 (4488) | 2019 (2367) | 1916 (3072) |
Notes: Values are presented as mean (standard deviation) or percentage, except for WMH (white matter hyperintensities) which are presented as median (interquartile range). APOE = Apolipoprotein E; BMI = Body mass index; Current smoker = Smoked tobacco products within the past 30 days; GMV = Gray matter volume; MMSE = Mini-Mental State Examination; SBP = Systolic blood pressure; TNF‐α = Tumor necrosis factor‐alpha.
*N = 421;
† N = 422;
‡ N = 409;
§ N = 392;
|| N = 217;
¶ N = 391;
# N = 322;
** N = 228;
†† N = 145.
Longitudinal Analyses
TNF‐α data were acquired at annual study visits over a time span of up to 8.4 years (mean = 2.2 years). There were no significant differences between participants who underwent one, two, or more than two study visits with respect to a number of characteristics (see Table 1), including baseline TNF‐α levels, age, sex, or apolipoprotein ε4 status (all ps >.05). However, participants with more than two study visits were somewhat more educated (mean education = 17.77) than participants with only one study visit (mean education = 17.20; p = .03). A sensitivity analysis demonstrated that excluding individuals with only one study visit did not meaningfully change any of our primary findings.
Longitudinal mixed-effects models demonstrated that TNF‐α levels significantly increased over time, correcting for baseline age, education, and sex (b = 0.02, z = 4.9, p ≤.001, n = 421). Linear within-person increases in TNF‐α were significantly associated with declines in GMV adjusting for baseline age, sex, and baseline TIV (b = −0.01, z = −3.84, p < .001, n = 290). The between-person effect was also significant (b = −0.005, z = −2.36, p = .02), indicating that individuals with greater mean levels of TNF‐α had smaller GMVs overall. Both the linear within-person effect (b = −0.01, z = −2.91, p = .004) and the between-person effect (b = −0.004, z = −1.97, p = .049) of TNF‐α on GMV remained significant upon including hs-CRP in the model as an additional covariate. hs-CRP was not significantly related to GMV (p = .92).
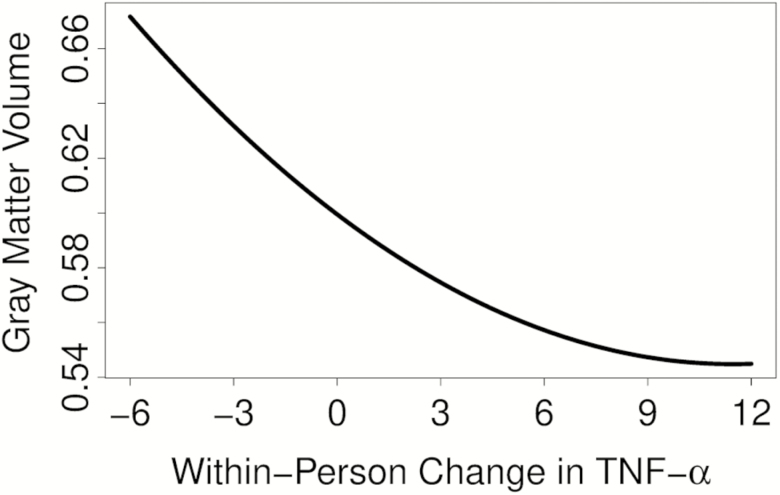
In the model testing for curvilinear effects, the quadratic within-person TNF‐α term (TNF‐α 2) was statistically significant (b = 0.0004, z = 3.16, p = .002), suggesting a nonlinear relationship between inflammation and GMV changes over time. Specifically, initial increases in TNF‐α appeared to be associated with more precipitous declines in GMV, which eventually plateaued in higher inflammatory states (see Figure 1). The curvilinear effect of TNF‐α 2 on GMV remained significant when also including hs-CRP in the model as a covariate (b = 0.001, z = 3.30, p = .001).
Figure 1.
Curvilinear relationship of within-person changes in TNF‐α to total GMV. To visualize the quadratic effect, the mixed-effects model was refit with all predictors centered except the within-person terms. Predicted values from that model were then plotted, thus displaying the curve of a subject with a typical (average) trajectory in the present sample. The x-axis depicts within-person change in TNF‐α (pg/mL) whereas the y-axis depicts corresponding total GMV values (L). As illustrated by the curve, initial increases in inflammatory status were associated with more precipitous GMV decline, which eventually plateaued. GMV = Gray matter volume; TNF‐α = Tumor necrosis factor‐alpha.
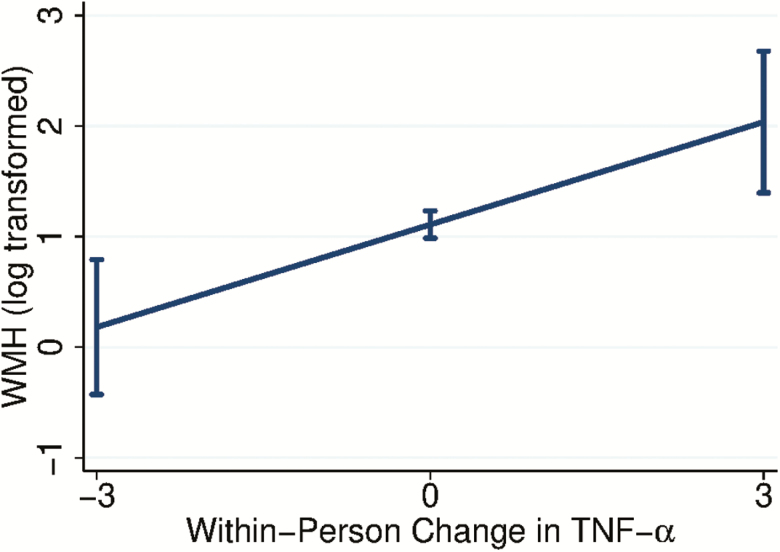
As illustrated in Figure 2, linear within-person increases in TNF‐α were significantly associated with increases in WMH adjusting for baseline age, sex, and baseline TIV (b = 0.31, z = 2.96, p = .003, n = 197). The between-person effect was not statistically significant (p = .81) and there did not appear to be a quadratic relationship between within-person changes in TNF‐α and WMH (p = .75). The linear within-person effect of TNF‐α on WMH remained significant when including hs-CRP in the model as an additional covariate (b = 0.29, z = 2.80, p = .01). hs-CRP was unrelated to WMH (p = .981).
Figure 2.
Linear relationship of within-person changes in TNF‐α to WMH. The x-axis depicts within-person change in TNF‐α (pg/mL) whereas the y-axis depicts corresponding WMH volumes (logarithmically transformed to meet assumptions of normality) with 95% confidence intervals. As illustrated in the graph, increases in TNF‐α over time were significantly associated with increases in WMH burden. TNF‐α = Tumor necrosis factor‐alpha; WMH = White matter hyperintensity.
In regard to cognitive outcomes, MMSE (b = −0.002, z = −4.63, p ≤ .001) and processing speed (b = 0.06, z = 3.64, p < .001) showed significant declines over time after controlling for baseline age, education, and sex. By contrast, episodic memory (b = 0.02, z = 3.25, p = .001) and executive functioning (b = 0.01, z = 2.21, p = .03) evidenced modest improvements over time, possibly related to practice effects (39). There was a significant linear relationship between within-person increases in TNF‐α and declines in MMSE scores adjusting for baseline age, sex, and education (b = −0.003, z = −2.1, p = .036, n = 409). The between-person effect was not statistically significant (p = .89). Within-person increases in TNF‐α continued to significantly relate to declines in MMSE scores upon also adjusting for hs-CRP (b = −0.008, z = 2.77, p = .01). hs-CRP was not significantly related to MMSE performance (p = .57).
Within-person changes in TNF‐α were not significantly related to changes in the executive functioning (b = 0.02, z = 0.92, p = .36, n = 409), episodic memory (b = 0.006, z = 0.17, p = .87, n = 399), or processing speed (b = −0.03, z = −.53, p = .6, n = 289) composite scores upon adjusting for baseline age, education, and sex. Follow-up analyses were conducted to evaluate the relationship of TNF‐α to performance on individual tasks within the composites that are particularly sensitive to brain aging and neurodegeneration, including phonemic fluency and California Verbal Learning Test, second edition, delayed free recall. Linear within-person changes in TNF‐α were not significantly related to changes in phonemic fluency performance (b = −0.08, z = −0.62, p = .53), but the between-person effect was statistically significant (b = −0.37, z = −2.34, p = .02), indicating that individuals with higher mean levels of TNF‐α had worse phonemic fluency overall. Neither the linear within-person effect (b = −0.13, z = −0.91, p = .36) nor the between-person effect (b = −0.20, z = −1.94, p = .052) of TNF‐α on California Verbal Learning Test, second edition delayed free recall was statistically significant, though the latter was at trend level in the expected direction. There were no significant quadratic relationships between TNF‐α and any of the cognitive outcomes (all ps >.05).
Influence of Vascular Risk Factors
The overall pattern of our primary findings was preserved upon additionally adjusting for vascular risk factors, including HbA1c, systolic blood pressure, and body mass index. More specifically, both the linear within-person effect (b = −0.01, z = −2.47, p = .01) and the between-person effect (b = −0.01, z = −2.18, p = .03) of TNF‐α on total GMV remained statistically significant when including vascular risk factors in the model. The curvilinear relationship between TNF‐α 2 and GMV was also upheld (b = 0.001, z = 3.20, p = .001). In addition, linear within-person increases in TNF‐α remained significantly associated with increases in WMH (b = 0.42, z = 2.70, p = .01). There continued to be a significant linear relationship between within-person increases in TNF‐α and declines in MMSE scores after adjusting for vascular risk factors (b = −0.01, z = −2.52, p = .012).
Post Hoc Analysis of Brain Regional Effects
Significant, linear relationships were observed between within-person increases in TNF‐α and gray matter volumetric declines in the default mode network (b = −353.46, z = −3.48, p ≤.001, n = 277), frontoparietal/executive network (b = −1129.64, z = −3.95, p ≤ .001, n = 277), dorsal attention network (b = −377.83, z = −3.61, p ≤.001, n = 277), and hippocampal network (b = −83.07, z = −3.58, p < .001, n = 277). Linear within-person increases in TNF‐α were also significantly associated with declines in bilateral hippocampal volumes (b = −0.06, z = −3.16, p = .002). The linear relation between within-person changes in TNF‐α and subcortical network volume approached but did not reach statistical significance in the expected direction (b = −90.99, z = −1.71, p = .09, n = 277). Upon including quadratic terms in the models, significant curvilinear relationships were observed between within-person changes in TNF‐α 2 and default mode network (b = 31.91, z = 3.59, p ≤.001), dorsal attention network (b = 34.70, z = 3.26, p = .001), hippocampal network (b = 6.70, z = 3.32, p = .001), and frontoparietal/executive network (b = 96.70, z = 3.65, p ≤ .001) but not with the subcortical network (p > .05). There was also a significant curvilinear relationship between within-person changes in TNF‐α 2 and bilateral hippocampal volumes (b = 0.005, z = 2.62, p = .01). All models were adjusted for baseline age, sex, and baseline TIV.
Discussion
The present findings demonstrate that aging is longitudinally associated with increases in peripheral levels of TNF‐α, a pro-inflammatory cytokine and master regulator of the innate immune response. In turn, within-person increases in plasma TNF‐α were associated with greater gray matter loss, WMH accumulation, and declines in global cognitive status over time, even within a functionally normal cohort. TNF‐α accounted for variance in these outcomes above and beyond vascular risk factors and a more routinely used peripheral marker of inflammation (hs-CRP). Interestingly, the longitudinal relationship between peripheral TNF‐α and GMV was curvilinear, such that initial increases in pro-inflammatory status were associated with more precipitous atrophy that eventually plateaued. Taken together, these results help to characterize the natural course of inflammatory changes in late life and suggest that elevated systemic TNF‐α may serve as a biomarker and mechanism of age-related declines in brain health.
The potential mechanisms underlying the relation between increased TNF‐α and GMV loss remain to be fully elucidated. In brain slice culture studies, it has been demonstrated that elevated TNF‐α blocks glutamate transporter activity and inhibits glutamate uptake, ultimately inducing neurodegeneration through glutamate neurotoxicity (40). Relatedly, elevated TNF‐α has also been associated with reduced synaptic plasticity and density, which over time may manifest as gray matter reductions (41). TNF‐α can additionally alter metabolism of the beta-amyloid precursor protein and, together with interferon gamma, facilitate production of beta-amyloid peptides while inhibiting secretion of soluble amyloid precursor proteins (42). In turn, beta-amyloid has been shown to overstimulate microglia and monocytes, leading to further increases in TNF‐α and associated neurotoxicity related to oxidative stress and apoptosis (43).
Regardless of the precise mechanism(s) involved, the curvilinear association between increases in TNF‐α and gray matter declines suggests that initial stages of TNF‐α-mediated inflammation may exert the largest effects on atrophy. Beyond a certain point of inflammation (see Figure 1), the effects of TNF‐α on brain volume seem to level off, possibly reflecting a saturation effect. Clinically, this supports the importance of early (or preventative) interventions to restore balance between pro- and anti-inflammatory cytokines, such as anti-inflammatory rich diets and physical exercise (44–46). Exploratory analyses suggested that the curvilinear relationship between TNF‐α and GMV was rather widespread in the brain and present across several established networks, including default mode network, dorsal attention network, hippocampal network, and frontoparietal/executive network. The exception was within the subcortical network composed of the basal ganglia and thalamus, suggesting that TNF‐α may be less associated with volumetric reductions in these regions. Although not statistically significant, the relationship was still in the expected direction.
Interestingly, longitudinal changes in TNF‐α were significantly related to changes in global cognition, as measured by the MMSE, but not to composite measures of episodic memory, processing speed, or executive functioning. Follow-up analyses, however, indicated that individuals with higher mean levels of TNF‐α showed worse verbal fluency performance, suggesting the possibility of nuanced relations to certain aspects of cognition.
In addition to relating to GMV, within-person increases in TNF‐α were significantly associated with increases in WMH burden. As with gray matter, the mechanisms underlying this relationship in humans remains an open question, though in vitro studies suggest that elevated TNF‐α causally contributes to necrosis of oligodendrocytes and demyelination (47). In addition, it has been established in the literature that TNF‐α is involved in the pathophysiology of cardiovascular disease (48), a major driver of white matter changes within the brain. In this study, however, longitudinal associations between TNF‐α and WMH, as well as other primary outcomes of interest, remained significant after controlling for vascular risk factors. This suggests the possibility of alternative pathways linking peripheral inflammation and adverse brain changes in aging adults.
There are limitations to this study that warrant mention. The number of study visits varied across participants and those with more than two visits were somewhat more educated than those with only one visit. Differences in education, and potentially other characteristics, between participants with varying numbers of study visits may have influenced our results. Moreover, the sample was high functioning, racially uniform (Caucasian), and likely possessed considerable reserve, particularly considering that the average educational level exceeded a college degree. This may have contributed to the observation that our episodic memory and executive functioning composites evidenced slight improvements over time, possibly due to practice effects (39). A different pattern of results may emerge in individuals characterized by greater diversity and variability in cognitive status, especially on gross cognitive screeners such as the MMSE which have clear ceiling effects and an overall restricted range in high-functioning populations like the present sample. In addition, it should be kept in mind that this study did not consider lifetime inflammatory status; the relation between TNF‐α and brain health may be influenced by prior histories of inflammation occurring earlier in the life span. Finally, the present analyses were correlational in nature and thus it is not possible to verify directionality of the observed effects. Although we hypothesize that TNF‐α-mediated inflammation contributes causally to brain changes, it is certainly possible that inflammation is better conceptualized as a biomarker of adverse brain changes rather than a driving mechanism. In a similar vein, TNF‐α was measured in the periphery, and the directionality of the relationship between the peripheral immune response and the central immune response remains to be clarified. Although it is possible that elevated TNF‐α in the periphery is a byproduct of elevated inflammation with the central nervous system, there is also research demonstrating that activation of the peripheral immune response leads to an exaggerated immune response in the brain (15). Although a correlation has been established in prior work between inflammatory markers in peripheral and central nervous systems (14), the directional (or bidirectional) nature of these relations remain to be fully established. Further complicating interpretation of the present findings is that we did not control for the use of anti-inflammatory or other medications that may have altered peripheral TNF‐α levels and influenced our results. This should be addressed in future research.
Despite these limitations, this study addresses an important gap in the literature by offering the first longitudinal analysis of the relation between systemic TNF‐α levels and brain health outcomes in a clinically normal older adult population. The finding that plasma TNF‐α concentrations significantly relate to changes in GMV, WMH burden, and global cognition suggests that inflammation in the periphery may reflect an important mechanism by which aging affects brain health over time. Our findings additionally support the use of blood-based measures of peripheral inflammation as relatively inexpensive and accessible biomarkers of adverse changes in gray and white matter in late life.
Funding
This work was supported by the National Institutes of Health-National Institute on Aging (UCSF ADRC P50 AG023501, R01AG032289, and R01AG048234 to J.H.K.; 23 AG058752 to K.B.C) and the Larry L. Hillblom Foundation (2014-A-004-NET and 2018-A-006-NET to J.H.K.; 2018-A-025-FEL to A.M.S.).
Conflict of Interest
J.H.K. helped develop the Delis–Kaplan Executive Function System and the California Verbal Learning Test, and thus receives royalties from Pearson Education, Inc. No other study authors have conflicts of interest.
Supplementary Material
References
- 1. Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 2. McNerlan SE, Ross OA, Maeve Rea I.. Cytokine Expression and Production Changes in Very Old Age. Cham, Switzerland: Springer International Publishing; 2018. doi: 10.1007/978-3-319-64597-1_40-1 [Google Scholar]
- 3. Fougère B, Boulanger E, Nourhashémi F, Guyonnet S, Cesari M. Chronic inflammation: accelerator of biological aging. J Gerontol A Biol Sci Med Sci. 2016;72:1218–1225. doi: 10.1093/gerona/glw240 [DOI] [PubMed] [Google Scholar]
- 4. Fenn AM, Norden DM, Godbout JP.. Neuroinflammation in Aging. Cham, Switzerland: Springer International Publishing; 2015. doi: 10.1002/9781118732748.ch6 [Google Scholar]
- 5. Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chi GC, Fitzpatrick AL, Sharma M, Jenny NS, Lopez OL, DeKosky ST. Inflammatory biomarkers predict domain-specific cognitive decline in older adults. J Gerontol A Biol Sci Med Sci. 2016;72:796–803. doi: 10.1093/gerona/glw155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wanigatunga AA, Varadhan R, Simonsick EM, et al. Longitudinal relationship between interleukin-6 and perceived fatigability among well-functioning adults in mid-to-late life. J Gerontol A Biol Sci Med Sci. 2018;74:720–725. doi: 10.1093/gerona/gly120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cauley JA, Manini TM, Lovato L, et al. The Enabling Reduction of Low-grade Inflammation in Seniors (ENRGISE) Pilot Study: screening methods and recruitment results. J Gerontol A Biol Sci Med Sci. 2019;74:1296–1302. doi: 10.1093/gerona/gly204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9:1245–1250. doi: 10.1038/nm939 [DOI] [PubMed] [Google Scholar]
- 10. Tarkowski E, Liljeroth AM, Minthon L, Tarkowski A, Wallin A, Blennow K. Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res Bull. 2003;61:255–260. doi: 10.1016/s0361-9230(03)00088-1 [DOI] [PubMed] [Google Scholar]
- 11. Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laws SM, Perneczky R, Wagenpfeil S, et al. TNF polymorphisms in Alzheimer disease and functional implications on CSF beta-amyloid levels. Hum Mutat. 2005;26:29–35. doi: 10.1002/humu.20180 [DOI] [PubMed] [Google Scholar]
- 13. Ekert JO, Gould RL, Reynolds G, Howard RJ. TNF alpha inhibitors in Alzheimer's disease: a systematic review. Int J Geriatr Psychiatry. 2018;33:688–694. doi: 10.1002/gps.4871 [DOI] [PubMed] [Google Scholar]
- 14. Bettcher BM, Johnson SC, Fitch R, et al. Cerebrospinal fluid and plasma levels of inflammation differentially relate to CNS markers of Alzheimer's disease pathology and neuronal damage. J Alzheimers Dis. 2018;62:385–397. doi: 10.3233/JAD-170602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wennberg AM V, Hagen CE, Machulda MM, Knopman DS, Petersen RC, Mielke MM. The cross-sectional and longitudinal associations between IL-6, IL-10, and TNFα and cognitive outcomes in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2019;74:1289–1295. doi: 10.1093/gerona/gly217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Sachdev PS, Wen W, et al. The relationship between inflammatory markers and voxel-based gray matter volumes in nondemented older adults. Neurobiol Aging. 2016;37:138–146. doi: 10.1016/j.neurobiolaging.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 18. Papenberg G, Ferencz B, Mangialasche F, et al. Physical activity and inflammation: effects on gray-matter volume and cognitive decline in aging. Hum Brain Mapp. 2016;37:3462–3473. doi: 10.1002/hbm.23252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smagula SF, Lotrich FE, Aizenstein HJ, et al. Immunological biomarkers associated with brain structure and executive function in late-life depression: exploratory pilot study. Int J Geriatr Psychiatry. 2017;32:692–699. doi: 10.1002/gps.4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 22. Casaletto KB, Staffaroni AM, Elahi F, et al. Perceived stress is associated with accelerated monocyte/macrophage aging trajectories in clinically normal adults. Am J Geriatr Psychiatry. 2018;26:952–963. doi: 10.1016/j.jagp.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casaletto KB, Elahi FM, Fitch R, et al. A comparison of biofluid cytokine markers across platform technologies: correspondence or divergence? Cytokine. 2018;111:481–489. doi: 10.1016/j.cyto.2018.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- 25. Friston K, Ashburner J, Kiebel S, Nichols T, Penny W.. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London, UK: Academic Press; 2011. [Google Scholar]
- 26. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/J.NEUROIMAGE.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 27. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 28. Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. doi: 10.1007/s12021-011-9109-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dadar M, Pascoal TA, Manitsirikul S, et al. Validation of a regression technique for segmentation of white matter hyperintensities in Alzheimer's disease. IEEE Trans Med Imaging. 2017;36:1758–1768. doi: 10.1109/TMI.2017.2693978 [DOI] [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 31. Kerchner GA, Racine CA, Hale S, et al. Cognitive processing speed in older adults: relationship with white matter integrity. PLoS One. 2012;7:e50425. doi: 10.1371/journal.pone.0050425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Staffaroni AM, Brown JA, Casaletto KB, et al. The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J Neurosci. 2018;38:2809–2817. doi: 10.1523/JNEUROSCI.3067-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002 [DOI] [PubMed] [Google Scholar]
- 34. Delis D, Kramer J, Kaplan E, Ober B.. California Verbal Learning Test. 2nd ed San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 35. Neuhaus JM, McCulloch CE. Separating between- and within-cluster covariate effects by using conditional and partitioning methods. J R Stat Soc Ser B (Statistical Methodol). 2006;68:859–872. doi: 10.1111/j.1467-9868.2006.00570.x [Google Scholar]
- 36. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26:3508–3526. doi: 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Power JD, Cohen AL, Nelson SM, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27:248–261. doi: 10.1093/arclin/acr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 41. Pickering M, Cumiskey D, O'Connor JJ. Actions of TNF-alpha on glutamatergic synaptic transmission in the central nervous system. Exp Physiol. 2005;90:663–670. doi: 10.1113/expphysiol.2005.030734 [DOI] [PubMed] [Google Scholar]
- 42. Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNFalpha plus IFNgamma induce the production of Alzheimer beta-amyloid peptides and decrease the secretion of APPs. FASEB J. 1999;13:63–68. doi: 10.1096/fasebj.13.1.63 [DOI] [PubMed] [Google Scholar]
- 43. Combs CK, Karlo JC, Kao SC, Landreth GE. Beta-amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/jneurosci.21-04-01179.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A. How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis. 2016;55:1–18. doi: 10.3233/JAD-160665 [DOI] [PubMed] [Google Scholar]
- 45. Feeney J, O'Leary N, Moran R, et al. Plasma lutein and zeaxanthin are associated with better cognitive function across multiple domains in a large population-based sample of older adults: findings from the Irish Longitudinal Study on Aging. J Gerontol A Biol Sci Med Sci. 2017;72:1431–1436. doi: 10.1093/gerona/glw330 [DOI] [PubMed] [Google Scholar]
- 46. Munguia L, Rubio-Gayosso I, Ramirez-Sanchez I, et al. High flavonoid cocoa supplement ameliorates plasma oxidative stress and inflammation levels while improving mobility and quality of life in older subjects: a double-blind randomized clinical trial. J Gerontol A Biol Sci Med Sci. 2019;74:1620–1627. doi: 10.1093/gerona/glz107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405 [DOI] [PubMed] [Google Scholar]
- 48. Svenungsson E, Fei GZ, Jensen-Urstad K, de Faire U, Hamsten A, Frostegard J. TNF-alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus. 2003;12:454–461. doi: 10.1191/0961203303lu412oa [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.