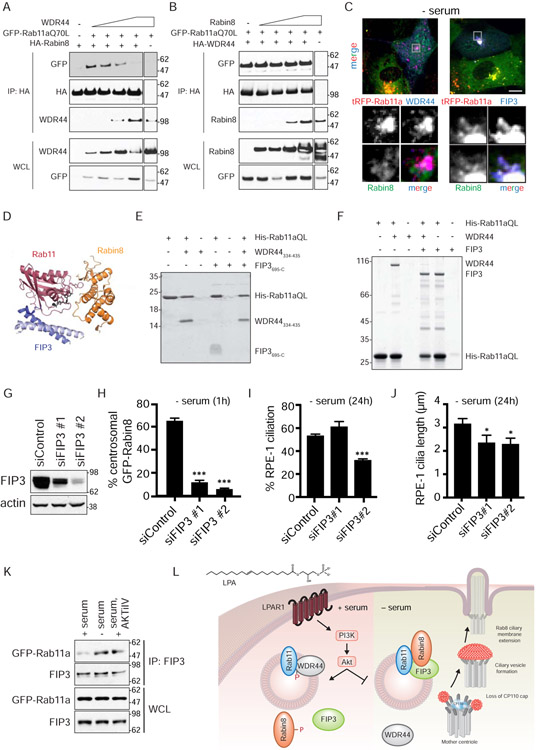
Figure 7: FIP3 functions in Rab11-dependent Rabin8 preciliary trafficking and ciliogenesis and competes with WDR44 for binding to Rab11a.
(A) Immunoblot showing co-immunoprecipitation of GFP-Rab11a Q70L with HA-Rabin8 in the presence of increasing amounts of WDR44 transiently-expressed in 293T cells grown in serum for 48h. Immunoblots were probed with GFP, HA, and WDR44 antibodies. Representative blot from two independent experiments is shown.
(B) Immunoblot showing co-immunoprecipitation of GFP-Rab11a Q70L with HA-WDR44 in the presence of increasing amount of Rabin8 as described in (A). Immunoblots were probed as described in (A). Representative blot from two independent experiments is shown.
(C) Immunostaining of RPE-1 cells stably-expressing GFP-Rabin8, tRFP-Rab11a, and HA-WDR44 or HA-FIP3 fixed with PFA and stained with HA antibodies. Grey box in top panels corresponds to the region of the cell in the bottom panels. Cells were serum-starved for 1h to promote Rabin8 trafficking and imaged by epifluorescence microscopy. Scale bar = 5μm.
(D) Structure of ternary complex containing Rab11, Rabin8, and FIP3 (Vetter et al., 2015).
(E) Coomassie-stained gel showing a Ni-NTA pull-down of WDR44334-435 (Rab11-binding domain of WDR44) and FIP3695-C (Rab11-binding domain of FIP3) by His-tagged Rab11aQ70L6-186 (HIS) (constitutively-active GTPase domain of Rab11a). Ni-NTA His-Rab11a Q70L6-186 (HIS) beads were incubated with and without excess FIP3695-C (308.7μM) and/or WDR44334-435 (354.4μM).
(F) Coomassie-stained gel showing a Ni-NTA pull-down as described in (E) using full-length WDR44 and FIP3. Ni-NTA His-Rab11a Q70L6-186 (HIS) beads were incubated with and without excess FIP3 (46μM) and/or WDR44334-435 (45μM).
(G) Western blot analysis of lysates from 293 cells treated with FIP3 siRNAs. Lysates were collected 72h after siRNA transfection. FIP3 and actin antibodies were used for immunoblotting.
(H) Quantification of GFP-Rabin8 centrosomal accumulation in RPE-1 cells upon treatment with FIP3 siRNA after 72h as described in Fig 1F. Mean ± s.e.m from three independent experiment are shown. >60 cells counted per treatment. ***P < 0.001.
(I) Quantification of ciliation in cells treated with FIP3 siRNAs for 72h and serum-starved for the final 24h. Cells were stained and imaged as described in Fig 1A. Mean ± s.e.m from three independent experiments is shown. ***P < 0.001.
(J) Quantification of cilia length in siControl and siFIP3-treated RPE-1 cells imaged as described in (H). *P < 0.05.
(K) Immunoprecipitation of endogenous FIP3 from GFP-Rab11a RPE-1 cells grown in 2% serum, 2% serum with AKTiIV (50nM), or serum-starved for 3h. FIP3 and GFP antibodies were used for immunoblotting. Representative immunoblots from three independent experiments for + serum and − serum, and two independent experiments for AKTiIV treatment are shown.
(L) Model for signaling regulation of the ciliogenesis Rab11-effector switch.
See also Figure S7.