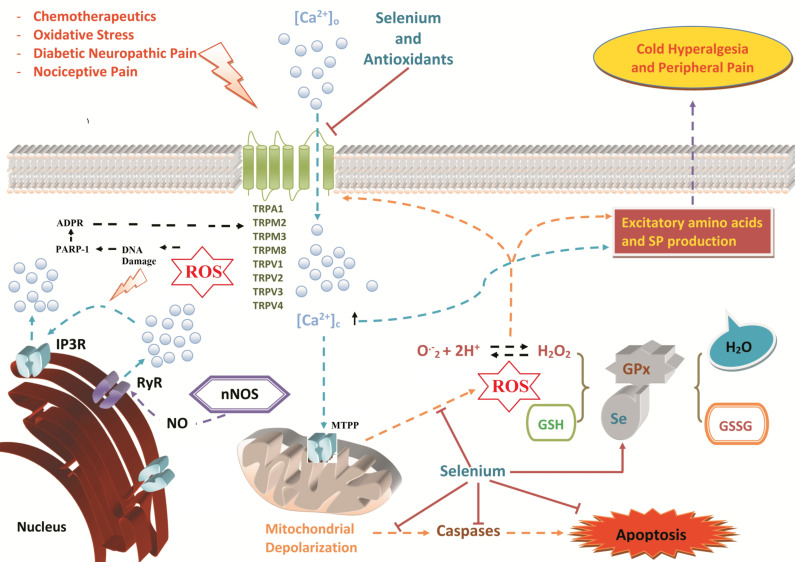
Fig. (1).
Role of selenium supplementation in TRP channel-mediated pathological mechanisms in neurons. Neural tissues are very vulnerable to oxidative stress-dependent cell death because of their lipid-rich structures. Many TRP channels (TRPA1, TRPC5, TRPM2, and TRPV1) are expressed in neuronal tissues and have unique functional and structural properties [3, 199]. Other neural TRP channels (TRPM3, TRPM8, TRPV2, TRPV3, and TRPV4) act as thermal sensors to hot or cold temperature. Chemotherapeutic agents, oxidative stress, diabetic neuropathic pain, and inflammatory and nociceptive pain may stimulate TRP channels to rapidly increase cytosolic Ca2+ concentrations and trigger calcium-activated calcium channel (CACC) entry. Overloading of cytosolic Ca2+ concentrations results in depolarization of the mitochondria via the opening of mitochondria transition permeability pores (MTPP). As a result of mitochondrial dysfunction, either caspase activity may result in apoptosis, or ROS overproduction activates oxidative stress-sensitive TRP channels. Excessive Ca2+ entry and ROS stimulate the release of pain mediators, such as substance P and other excitatory amino acids, causing PKC activation, NO synthesis, RyR activation and IP3 receptor activation (IP3R) [13]. TRPM2 channel is activated in the peripheral neurons by ROS-induced DNA damage and ADP-ribose (ADPR) production through PARP-1 enzyme activation. Several reports have concluded that selenium (Se) inhibits the activation of TRP channels and Ca2+ signaling, as well as decreases mitochondrial depolarization and ROS overproduction. Supplementation with selenium prevents apoptotic cell death by reducing caspase 3 and 9 activities. Selenium is also a cofactor for the antioxidant GPx in reducing excessive ROS production in cytosolic compartments. (A higher resolution / colour version of this figure is available in the electronic copy of the article).