Abstract
Uncertainty about the causes of neonatal deaths impedes achieving global health targets to reduce mortality. Complete diagnostic autopsy (CDA) is the gold standard to determine cause of death. However, it is often difficult to perform in high-burden, low-income settings. Validations of more feasible methods to determine cause of death are needed. This prospective, multi-center study in Ethiopia assessed the validity of the minimally invasive tissue sampling (MITS) approach to contribute to causes of death in preterm neonates compared to CDA. The MITS and CDA of 105 cases were reviewed. The MITS sampling success for lungs and liver was 100% and 84%, respectively. The kidney and brain had sampling successes of 58% each. MITS showed good agreement with CDA for the diagnosis of hyaline membrane disease (kappa = 0.78), and moderate to substantial agreement for pneumonia and pulmonary hemorrhage (kappa = 0.59 and 0.68, respectively). Even though CDA is the gold standard in identifying the cause of death, we believe that the MITS method can be a useful alternative method in supporting determination of cause of death in low-resource settings.
Keywords: autopsy, newborn mortality, Ethiopia, MITS
Introduction
In 2017, 2.5 million newborn deaths occurred globally accounting for 47% of under-five deaths.1 About one-third of these infants died as a result of preterm-related causes and 99% occurred in low- and middle-income countries (LMIC).1 Understanding the causes and factors contributing to preterm deaths is needed to identify and inform interventions targeted to reduce the burden of mortality. This is especially true in low-resource settings, where the cause of death (COD) is often not recorded and when recorded, is often based on a physician’s opinion or verbal autopsy, a structured interview of a family member of the deceased about the signs and symptoms of disease experienced before death.2 Both clinical opinion and verbal autopsy often provide inaccurate attribution of COD.2-4
Complete diagnostic autopsy (CDA) is considered the “gold standard” method for determining the attribution of COD. In addition to its role in medical education, it provides accurate COD determination for the purposes of mortality surveillance.5 However, CDA is rarely practiced in low-resource settings and over the years, an overall decline in autopsy rates has been reported even in high-resource settings. For example, prior to 1970, autopsy rates among hospital deaths in the United States were 40% to 60%,6-8 which have declined to about 5% in recent years. Reports from Australia, Denmark and Netherlands show similar trends of CDA decline in recent decades.8-10 Evidence continues to show that, in the absence of CDA, the COD certification is often inaccurate11 even when other diagnostic modalities are readily available.12
Minimally invasive tissue sampling (MITS), a possible alternative to the CDA,5 involves systematic tissue sampling that is designed to contribute to COD determination. It may be especially useful in high mortality settings, where CDA is not feasible due to costs, man-power constraints, and other technical considerations.13 MITS validity and usefulness have been assessed in different settings and with different protocols that incorporate core biopsy samples for histopathology diagnosis, and body tissue, fluid or blood samples for microbiology, and different imaging modalities.14,15 While MITS has been validated against CDA in adults and older children, there is limited experience in neonates and especially in preterm infants.16
As part of a larger study on COD among preterm infants in Ethiopia,17 this paper aims to assess the sampling success and the validity of MITS when compared to CDA in a low-resource setting.
Methods
Study Setting and Design
The study was conducted as part of the Study of Causes of Illness and Death in Preterm Infants in Ethiopia (SIP Study).17 It was a prospective multi-center observational study conducted in 5 hospitals across 3 geographic regions in Ethiopia from July 2016 to May 2018. Details are published elsewhere.20
All preterm infants admitted to any one of the study hospitals with a gestational age of less than 37 completed weeks and a postnatal age of <7 days were screened and if eligible and consented, enrolled. Three methods (ultrasound, last menstrual period and physical examination using the new Ballard Score) were used to estimate gestational age and to confirm that an infant was less than 37 weeks. Any delivery which was a result of an induced abortion or for which the gestational age could not be reliably determined using study criteria was excluded.
Ethical Approvals
The study protocol was approved by the ethical review committees of participating institutions in Ethiopia (Addis Ababa University’s Tikur Anbessa and Ghandi Memorial hospitals, Jimma University, the University of Gondar, and St Paul Hospital Millennium College). All clinical procedures were conducted per hospital protocol and informed consent (written) was taken from parents prior to their participation in the study as well as separate consent (written) for CDA and /or MITS in the event of a neonatal death. (AAU IRB approval number AAUMF 03-008)
MITS Procedures
Pre-structured standard operating procedures were followed. All pathologists involved in the study completed training on the basic concepts of MITS and successfully performed practical demonstrations on sample collection prior to commencement of the MITS data collection. The MITS procedures were introduced after the CDA because of logistical challenges obtaining the MITS needles and related supplies.
For each participant with confirmed consent, a MITS was performed using a prepared kit consisting of a Bard monopty biopsy needle 16G, 100 mm, placemat, measuring tape and 5 formalin jars. After anthropometric measurements were taken and a thorough external examination was performed, punctures were made on targeted organs. Approximately 6 samples of tissue were collected from each lung, 3 samples from the liver, 2 samples from each kidney and 6 samples from the brain using the trans-nasal and trans-fontanel approach. The detailed techniques for the MITS method are described elsewhere.18
CDA Procedures
For the CDA procedures, pathologists followed standard autopsy protocols which included anthropometric measurements, documenting external gross abnormalities, organs in the thorax (both lungs, trachea, heart, pericardium), in the abdomen (small and large intestines, liver, pancreas, spleen, both kidneys, adrenal glands), genital organs, blood vessels, skull, and brain (periventricular, cortical and cerebellar areas). After dissection, samples were fixed and embedded for histologic examination.
Histological Analyses
Tissue specimens were fixed in 10% neutral buffered formalin for 24 hours and stained with hematoxylin and eosin as per standard procedures. No microbiological studies were done on either CDA or MITS samples.
Data Collection, Entry and Analysis
Both the CDA and MITS results were reported using a standardized format. The format included a checklist of major preterm conditions and expected histologic features for each. The pathologist summarized the major findings. A team of one pathologist and one pathology resident reviewed and analyzed the samples from the MITS as well as the CDA slides. A single case could have 2 or more relevant pathologic findings that ultimately contributed to COD determination. The cause of death for each case was determined using available clinical, laboratory, MITS, and autopsy data by a panel of experts. The panel assigned the primary cause of death using ICD-10.17,31
Sampling success of the MITS method was defined as the presence of targeted organ tissue on embedded tissue, without any specific measurement of size or number required for adequacy. Cases with complete lack of reports or incomplete reports on the CDA or MITS done were excluded from further analysis.
Data were entered into study computers using the data management system developed for this study. The concordance between the diagnoses obtained by MITS and the CDA was assessed by the kappa statistic.19
The diagnostic accuracy of MITS to identify the categories established by the CDA diagnosis was evaluated as sensitivity, specificity, positive and negative predictive values. The sampling success of the MITS technique was also reported. The average amounts of time taken to complete MITS and CDA were calculated and presented as descriptive statistics.
Results
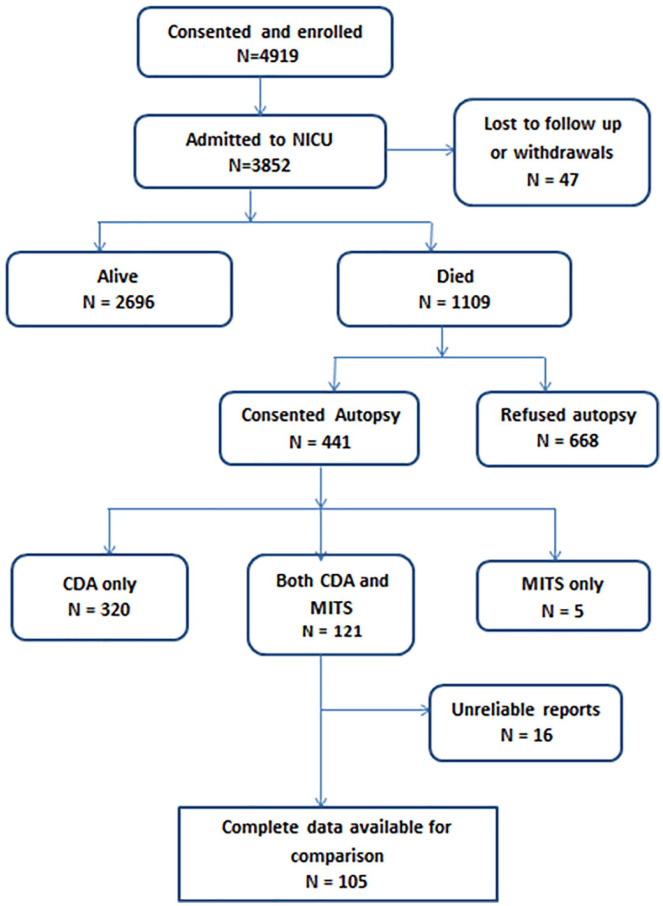
The total number of live-born preterm babies enrolled in the SIP study was 4919 (Figure 1). Of the 1109 (22.5%) infants enrolled who died, informed consent for autopsy was available for 441 (39.8%). Both MITS and CDA were performed in 121 (10.9%) of the deaths. Complete data for comparison of MITS with CDA were available in 105 cases (Figure 1). Data to determine the sampling yield of the MITS method was available for 91 cases (Table 1).
Figure 1.
Enrollment diagram.
Table 1.
Demographic and Clinical Characteristics of Study Population.
| Variables | Total deaths (1109) |
Consented to autopsy (441) | Did not consent to autopsy (663) | Both MITS and CDA (121) | Selected for comparison (105) |
|---|---|---|---|---|---|
| Gestational age | |||||
| <28 weeks | 89 (8.03%) | 35 (7.94%) | 54 (8.14%) | 10 (8.26%) | 10 (9.52%) |
| 28-31 weeks | 512 (46.17%) | 213 (48.30%) | 295 (44.49%) | 63 (52.07%) | 54 (51.43%) |
| 32-34 weeks | 349 (31.47%) | 134 (30.39%) | 214 (32.27%) | 28 (23.14%) | 22 (20.95%) |
| ≥35 weeks | 159 (14.34%) | 59 (13.38%) | 100 (15.08%) | 20 (16.53%) | 19 (18.10%) |
| Age at death (days) | |||||
| ≤ 7 days | 880 (79.35%) | 366 (82.99%) | 510 (76.92%) | 96 (79.34%) | 89 (84.76% |
| 8-14 days | 147 (13.25%) | 44 (9.97%) | 103 (15.53%) | 17 (14.05%) | 12 (11.43%) |
| 15-21 days | 40 (3.61%) | 16 (3.63%) | 24 (3.62%) | 2 (1.65%) | 2 (1.90%) |
| ≥22 days | 23 (2.07%) | 6 (1.36%) | 16 (2.41%) | 1 (0.83%) | 1 (0.95%) |
| Gender | |||||
| Female | 495 (44.63%) | 193 (43.76%) | 301 (45.39%) | 48 (39.67%) | 42 (40.00%) |
| Male | 598 (53.92%) | 243 (55.10%) | 351 (52.94%) | 72 (59.50%) | 62 (59.05%) |
| Mode of delivery | |||||
| Normal | 678 (61.14%) | 265 (60.09%) | 237 (35.75%) | 83 (68.59%) | 76 (72.38%) |
| Cesarean section | 404 (36.43%) | 167 (37.87%) | 408 (61.54%) | 38 (31.40%) | 29 (27.62%) |
| Location of delivery | |||||
| Home | 66 (5.9%) | 13 (2.95%) | 23 (3.47%) | 3 (2.48%) | 3 (2.86%) |
| Study hospital | 886 (79.9%) | 301 (68.25%) | 434 (65.46%) | 74 (61.16%) | 65 (61.90%) |
| Transferred | 157 (14.2%) | 118 (26.75%) | 187 (28.20%) | 41 (33.88%) | 34 (32.38%) |
| Maternal risk factor | |||||
| Multiple pregnancy | 325 (29.30%) | 133 (30.16%) | 195 (3.47%) | 34 (28.09%) | - |
| Maternal condition | |||||
| Diabetes | 11 (0.99%) | 4 (0.91%) | 7 (1.06%) | 0 | 0 |
| Hypertension | 309 (27.86%) | 133 (30.16%) | 175 (26.39%) | 34 (28.09%) | 30 (28.57%) |
| Cardiac condition | 9 (0.81%) | 7 (1.59%) | 2 (0.30%) | 0 | 0 |
| Maternal infection | |||||
| UTI | 56 (5.05%) | 13 (2.95%) | 43 (6.49%) | 3 (2.48%) | 2 (1.90%) |
| HIV serostatus | 23 (2.07%) | 13 (2.95%) | 10 (1.51%) | 2 (1.65%) | 1 (0.95%) |
| History of TB | 10 (0.90%) | 4 (0.91%) | 6 (0.90%) | 1 (0.83%) | 1 (0.95%) |
| Chorioamnionitis | 52 (4.69%) | 28 (6.35%) | 24 (3.62%) | 7 (5.78%) | 7 (6.67%) |
| PROM | 104 (9.38%) | 28 (6.35%) | 76 (11.46%) | 7 (5.78%) | 4 (3.81%) |
| Maternal fever | 79 (7.12%) | 31 (7.03%) | 48 (7.24%) | 7 (5.78%) | 7 (6.67%) |
| Birth weight | |||||
| <1000 g | 137 (12.35%) | 59 (13.38%) | 76 (11.46%) | 17 (14.05%) | 15 (14.29%) |
| 1000 to <1500 g | 482 (43.46%) | 201 (45.58%) | 280 (42.23%) | 58 (47.93%) | 49 (46.67%) |
| 1500 to <2000 g | 314 (28.31%) | 122 (27.66%) | 190 (28.66%) | 34 (28.10%) | 31 (29.52%) |
| ≥2000 g | 154 (13.89) | 48 (10.88) | 106 (15.99) | 8 (6.61) | 6 (5.71) |
Abbreviations: UTI, urinary tract infection; PROM, Premature rupture of membranes; TB, tuberculosis; HIV, Human Immunodeficiency Virus.
Comparison of Diagnoses Identified by CDA versus MITS
CDA identified pathological findings in 104/105 (99%) of cases. Of the tissues evaluated, most pathologic findings involved the lungs followed by the brain (Table 2). Major histopathologic findings contributing to death were identified in 89/105 (84.8%) of the cases using the MITS method. Sixteen cases were reported as non-conclusive using MITS. In one case, significant pathology could not be detected either by CDA or MITS.
Table 2.
Frequency of Major Pathologic Findings on CDA and MITS.
| FINDINGS | CDA (N = 106) | MITS (N = 106) | CDA finding also found by MITS (%) |
|---|---|---|---|
| Cardiac and respiratory findings | |||
| HMD | 65 | 60 | 92.3 |
| PH | 32 | 20 | 62.5 |
| Pneumonia | 17 | 8 | 47 |
| Aspiration | 1 | 1 | 100 |
| Pulmonary congestion | 1 | 0 | - |
| Interstitial emphysema | 1 | 0 | - |
| CHF secondary to TOF | 1 | 0 | - |
| Central nervous system findings | |||
| IVH | 17 | 2 | 11.8 |
| Meningitis | 10 | 4 | 40.0 |
| PNA/HIE | 12 | 4 | 33.3 |
| Kernicterus | 2 | 0 | - |
| Leigh syndrome | 1 | 0 | - |
| Subarachnoid hemorrhage | 1 | 0 | - |
| Gastrointestinal and Genitourinary findings | |||
| Hepatic injury | 1 | 0 | - |
| ATN | 3 | 1 | 33 |
| Splenic hemorrhage | 3 | 0 | - |
| NEC | 1 | 0 | - |
| Pyelonephritis | 1 | 1 | 100 |
| Others | |||
| Sepsis | 1 | 2 | - |
| Subgaleal hemorrhage | 1 | 1 | 100 |
| Non-conclusive | 1 | 18 | |
Abbreviations: HMD, hyaline membrane disease; PH, pulmonary hemorrhage; IVH, intraventricular hemorrhage; ATN, acute tubular necrosis; PNA, perinatal asphyxia; HIE, hypoxic-ischemic encephalopathy; NEC, necrotizing enterocolitis.
Diagnostic Accuracy of the MITS Method Compared to CDA as a Gold Standard
MITS showed substantial concordance with the CDA for the diagnosis of hyaline membrane disease and for pulmonary hemorrhage (kappa = 0.78 and 0.68, respectively) and moderate agreement for pneumonia (kappa = 0.59). There was only a slight concordance for intraventricular hemorrhage (kappa = 0.09; Table 3).
Table 3.
Sensitivity, Specificity, Positive and Negative Predictive Value (PPV and NPV), Accuracy and Kappa Value of MITS Compared to CDA for Major Pathologic Findings in 105 Cases.
| Variables | HMD | Pneumonia | Intraventricular Hemorrhage |
Pulmonary Hemorrhage |
|---|---|---|---|---|
| Sensitivity | 87.7% | 47.1% | 5.9% | 62.5% |
| Specificity | 92.5% | 100% | 100% | 98.6% |
| PPV | 86.4% | 100% | 100% | 95.2% |
| NPV | 70.6% | 90.7% | 84.6% | 85.7% |
| Kappa | 0.78 | 0.59 | 0.09 | 0.68 |
| Accuracy | 82.0% | 91.4% | 84.8% | 87.6% |
The MITS method showed 87.7% sensitivity, 92.5% specificity, 86.4% positive predictive value (PPV), and 70.6% negative predictive value (NPV) in diagnosing hyaline membrane disease with a kappa value of 0.78. There was moderate agreement (kappa of 0.59) between CDA and MITS in the diagnosis of pneumonia. MITS showed 47.1% sensitivity, 100% specificity, 100% PPV, and 90.7% NPV compared to the diagnosis by CDA. Other common pulmonary findings such as pulmonary hemorrhage also had substantial concordance between MITS and CDA (kappa of 0.68). Twenty out of the 32 cases of pulmonary hemorrhage were detected on MITS. Five of the twelve missed pulmonary hemorrhage cases had MITS reports that were not conclusive. The other remaining 7 cases had superimposed pneumonia and/or hyaline membrane disease.
Pulmonary pathology findings accounted for 67.6% (117/173) of all the major findings on CDA. MITS sampling was able to detect 89 of the 117 major pulmonary findings (76.1 %; Figure 2). However, MITS was only able to detect 2 out of 17 (11.8%) cases of IVH identified by CDA.
Figure 2.
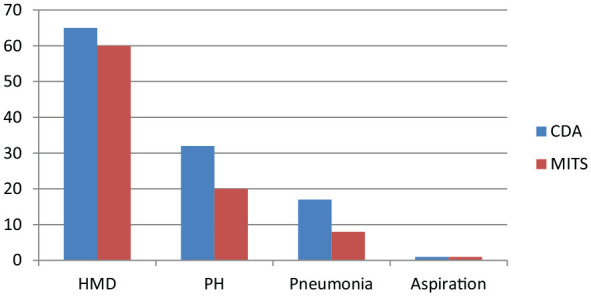
Comparison of frequency of major pulmonary findings as reported on CDA and MITS.
Abbreviations: PH, pulmonary hemorrhage; HMD, hyaline membrane disease; CDA, complete diagnostic autopsy; MITS, minimally invasive tissue sampling
The average time to perform the CDA was 67 minutes whereas the MITS procedure took an average of 28 minutes. MITS histologic slides were re-evaluated for 91 cases. The lungs had the highest proportion of adequate samples and yielded the most informative histopathologic findings, in particular for the diagnosis of hyaline membrane disease (Table 4).
Table 4.
Minimally Invasive Tissue Sampling Yield for Specific Organs.
| Organ | Yield (sampling success %) |
|---|---|
| Lung | 91/91 (100) |
| Liver | 75/89 (84) |
| Kidney | 52/90 (58) |
| Brain | 50/87 (58) |
| Spleen | 4/26 (15)* |
| Intestines | 4/26 (15)* |
Only one of the study hospitals reported minimal yield for spleen and intestines. Other hospitals reported no yield.
Figures 3 to 5 are examples of histological findings showing characteristic hyaline membranes of HMD with collapsed and air-filled alveoli in both MITS and CDA samplings. Figure 6 and 7 show gross and microscopic findings of intraventricular hemorrhage.
Figure 3.
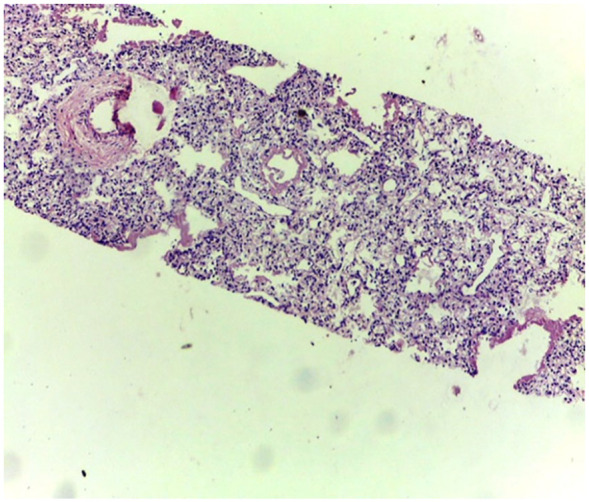
Low power view of lung biopsy obtained by MITS. Collapsed and air-filled alveoli are seen. Characteristic hyaline membranes of HMD is observed in more than few air spaces.
Figure 5.
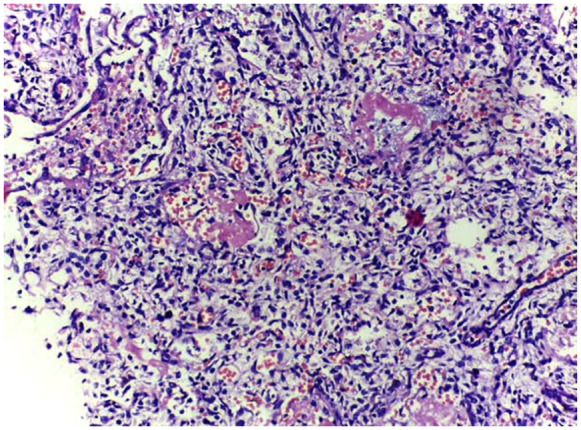
CDA sample of lung showing features of hyaline membrane disease and pulmonary hemorrhage.
Figure 6.
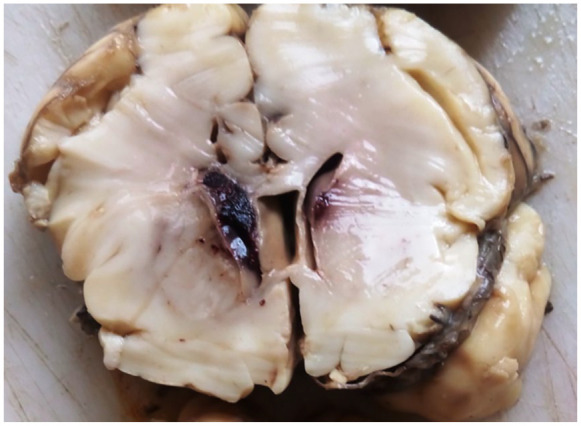
Old intraventricular hemorrhage as demonstrated on CDA.
Figure 7.
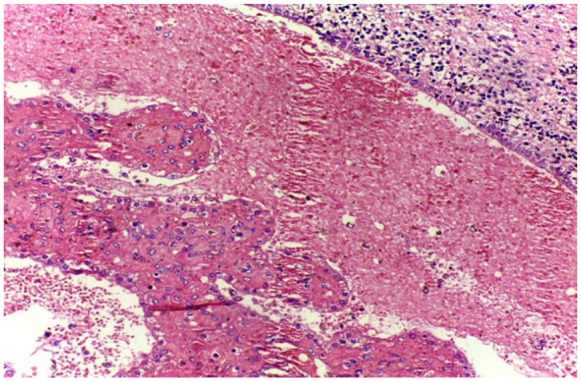
IVH on microscopy showing the simple cuboidal ependymal layer and extensive hemorrhage in the ventricles on CDA.
Figure 4.
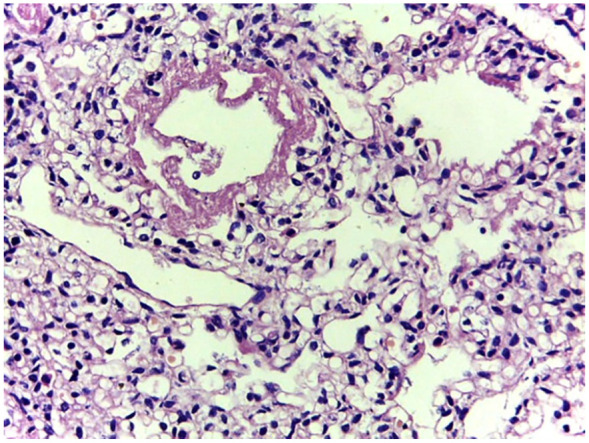
A closer look at the specimen depicted on Figure 3 shows the hyaline membranes and structural immaturity of air spaces in a specimen obtained by MITS.
Discussion
This is the among the first studies to evaluate the validity of a minimally invasive postmortem procedure in preterm neonates by comparing the MITS’ results with those achieved with CDA, the gold standard for COD determination.
The MITS procedure saved considerable time as it took about 39 minutes less than the CDA procedure. Sampling the lungs was very successful because the lungs are easily accessible and the depth at puncture can be estimated. More representative core specimens were collected from the lungs as compared to other organs. Similarly, the liver had a high sampling yield. Located at the right upper quadrant of the abdomen, close to the skin, and not obscured by other organs, it was not difficult to retrieve good samples from the liver. Studies involving neonates reported comparable liver sampling success ranging from 77% to 92%.27,28 In one of the studies, the liver was found to be the most diagnostically useful.28 In their study involving stillbirths, Breeze et al reported successful sampling of the lungs in 86% of cases.29 Adult studies reported a much better success sampling of the lungs (90-100%).18,30
Other abdominal organs such as the kidneys had a moderate sampling success; however, the MITS sampling success from deep abdominal organs such as the spleen and intestines was limited. MITS sampling of the brain through the trans-nasal and trans-fontanel approaches yielded a 58% success. While the sampling yield from the brain may be fair, the sampled tissues were usually very small and random, making them difficult to use for diagnostic interpretation. Localized changes of ischemia, kernicterus, or intraventricular hemorrhage (IVH) could not be detected in most cases. In addition, the brain tissue was difficult to handle as it was very friable and often got lost during tissue processing. Sampling the brain as early as possible after death and appropriate tissue fixation might have provided a better yield.
IVH was particularly difficult to assess because most samples from the brain were inadequate which could be due to the randomness of the sampling process. IVH was not difficult to diagnose with CDA because the blood in the ventricles and the brain could be appropriately evaluated to reflect these findings. IVH was mostly missed by MITS as it was difficult to target the periventricular area without the assistance of any imaging technique. Other less common central nervous system (CNS) findings such as kernicterus and subarachnoid hemorrhage would be expected to be missed using the MITS technique since both conditions require open procedures to observe changes.
Comparison of the 2 methods showed that the MITS diagnoses had substantial agreement for pulmonary hemorrhage and hyaline membrane disease with the CDA diagnoses. This validation study of the MITS method showed significant concordance between MITS and CDA for pulmonary findings.
Respiratory distress syndrome, with the typical pathologic findings of hyaline membrane disease, remains the most important cause of death in preterm neonates particularly in the developing world.21,22 In this study, CDA found that about 65% of the cases had this common prematurity condition either alone or with other findings such as pulmonary hemorrhage and pneumonia. In almost 88% of these cases, samples obtained using MITS identified features that were either diagnostic or strongly suggestive of hyaline membrane disease. MITS gave false positivity in 3 cases. The concordance between MITS and CDA regarding HMD diagnosis was substantial. A total of 8 cases of hyaline membrane disease were missed on MITS. Six of these cases had MITS reports that were not conclusive suggesting either that the tissue submitted was suboptimal for final diagnosis or no significant pathology could be detected with the sampled tissue. The other 2 cases had superimposed pulmonary hemorrhage as a complication. MITS reliably detected the pulmonary hemorrhage in both cases but failed to identify features of hyaline membrane disease. This result could be explained by the masking of underlying structural immaturity of the alveoli and characteristic hyaline membranes by extensive intra-alveolar hemorrhage.
The cases of pneumonia missed were either due to non-diagnostic MITS samples or due to superimposed pulmonary hemorrhage that masked some changes. In addition, pneumonia could be a focal process that could be missed on blind random sampling of the lungs. For such cases, particularly in the presence of clinical history suggesting a pulmonary infection, X-ray findings before making a lung puncture could help direct the biopsy needle to the lobe with expected pathology.
One of the limitations of our study was that neither CDA nor MITS used any microbiology to evaluate presence of pathogens to assess infectious causes. Another limitation was that, despite guidelines for both CDA and MITS, individual clinical judgment was still required which may have biased some of the findings. The sample size used to compare CDA and MITS was limited because some of the reports that needed to be filled by the pathologists were incomplete and could not be used for comparison.
The pediatric autopsy rates in developing countries are generally low as seen in Malaysia where the pediatric autopsy rate was 4.5%.23 A much lower rate was documented at the University of Benin teaching hospital in Nigeria where the neonatal autopsy rate was just 0.8%.24 Formally documented neonatal autopsy records are not readily available in Ethiopia. However, personal experience of the co-authors estimated that in Tikur Anbessa hospital, there were only about 40-50 autopsies done every year and most were stillbirths and neonatal deaths.
One of the reasons to explain such low rates of autopsy in developing countries is lack of or shortage of facilities and trained personnel to conduct CDAs.15 In Africa, practice of pathology services is not on the levels seen elsewhere in the world. Few African countries have pediatric pathology as a field of specialty.16 In addition to the limited availability of skilled pathologists in low-resourced settings, CDAs are quite costly. In their study, Weustink et al calculated a mean cost of $2274 (USD) per CDA.16,17 The invasive nature of the procedure also poses an obstacle to obtaining informed consent from family members, given cultural and religious considerations.8 These challenges force clinicians to look for cheaper, easier and more acceptable alternatives to the CDA.
A study conducted in a Mozambique quaternary hospital showed a substantial degree of concordance (89%, kappa value of 0.70) between MITS and CDA diagnosis in a series of 54 pediatric cases.19 MITS performed well in the detection of pediatric infection and cancers particularly when the disease was a disseminated one. The MITS method (also referred to as minimally invasive autopsy, in some studies) performed poorly for the detection of congenital malformations and non-infectious conditions. Similarly, in a series involving stillborn babies and neonates, the MITS method registered a high level of percentage of accuracy in all diagnostic categories (>75%).25 While MITS has been validated to some extent in adults and in neonates,26 there is limited literature regarding its application to preterm neonatal deaths.
We conclude that in the absence of evidence of chromosomal abnormalities, anatomic malformations, and other conditions that require open procedures, MITS can be used to reliably detect major findings that contribute to death in preterm infants. MITS performed well in detecting respiratory problems that contribute to most of the deaths in preterm infants such as respiratory distress syndrome, pneumonia and pulmonary hemorrhage. To this end, in instances involving deaths following respiratory symptoms, MITS could be sufficient to identify significant findings that contribute to death. However, CDA remains the gold standard method of determining COD particularly when infants die of multi-system causes.
We recommend further testing of the MITS method with inclusion of additional diagnostics such as cranial ultrasound and abdominal X-ray to detect some findings that could be missed because of blind and random sampling such as for NEC or IVH. Microbiologic studies could be a useful complement as neonatal infections are a major cause of death in this age group. We also recommend that the MITS procedures be further tested using lower-level health professionals such as technicians, clinical officers and nurses after appropriate training so that it can be used more widely in low resource settings.
Acknowledgments
We would like to acknowledge the financial and technical support from the Bill and Melinda Gates Foundation. We would like to acknowledge staff of the pathology departments of Jimma University hospital (TesfayeHurgesa and AbdoKedir); Addis Ababa University (MahletArayasellassie and TewodrosYalew), Gondar University hospital (LisanuMezgebu and ManegerewMuche) and of St Paul hospital (Aisha Jibril and TsionBetremariam) for their technical support. We would like to thank IsGlobal of Barcelona University for providing us with the MITS needles and training of our pathologists.
Footnotes
Author Contributions: LMM - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript.
AN - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript. AN contributed on his personal interest and not representing the Bill and Melinda Gates Foundation.
EMM - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript.
RLG - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript.
AM - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript.
MA - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript.
ZT - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript.
BE - conceived the study, contributed to the study design, data collection, data analysis, data interpretation and writing of the manuscript.
RH - contributed to performing the standard autopsy and MITS, data collection, data interpretation and writing of the manuscript.
TD - contributed to performing the standard autopsy and MITS, data collection, data interpretation and writing of the manuscript.
YB - contributed to performing the standard autopsy and MITS, data collection, data interpretation and writing of the manuscript.
TB - contributed to performing the standard autopsy and MITS, data collection, data interpretation and writing of the manuscript.
BA - contributed to performing the standard autopsy and MITS, data collection, data interpretation and writing of the manuscript.
MB - contributed to performing the standard autopsy and MITS, data collection, data interpretation and writing of the manuscript.
YG - contributed to performing the standard autopsy and MITS, data collection, data interpretation.
AA - contributed to performing the standard autopsy and MITS, data collection, data interpretation and writing of the manuscript.
All authors read and approved the final draft of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by grants from the Bill & Melinda Gates Foundation [grant number OPP1136965]
Ethical Approvals: The study protocol was approved by the ethical review committees of participating institutions in Ethiopia (Addis Ababa University’s Tikur Anbessa and Ghandi Memorial hospitals, Jimma University, the University of Gondar, and St Paul Hospital Millennium College). All clinical procedures were conducted per hospital protocol and informed consent (written) was taken from parents prior to their participation in the study as well as separate consent (written) for CDA and/or MITS in the event of a neonatal death. (AAU IRB approval number AAUMF 03-008)
ORCID iDs: Rahell Hailu  https://orcid.org/0000-0003-1711-3346
https://orcid.org/0000-0003-1711-3346
Beza Eshetu  https://orcid.org/0000-0001-5181-650X
https://orcid.org/0000-0001-5181-650X
Lulu M Muhe  https://orcid.org/0000-0002-2776-9923
https://orcid.org/0000-0002-2776-9923
References
- 1. UNICEF, WHO, World Bank, UN-DESA Population Division. Levels and trends in child mortality report 2017. Estimates Developed by the UN Inter-agency Group for Child Mortality Estimation. Updated September 2018. Accessed January 25, 2019 http://www.who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2017/en/
- 2. Nichols EK, Byass P, Chandramohan D, et al. The WHO 2016 verbal autopsy instrument: an international standard suitable for automated analysis by InterVA, InSilicoVA, and Tariff 2.0. PLoS Med. 2018;15:e1002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soleman N, Chandramohan D, Shibuya K. Verbal autopsy: current practices and challenges. Bull World Health Organ. 2006;84:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ganapathy SS, Khoo YY, Azahadi MO, Mohamad Anuar MF, Jeevananthan C, Rao C. Validation of verbal autopsy: determination of cause of deaths in Malaysia. BMC Public Health. 2017;17:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kaschula ROC. The Pediatric Autopsy in Africa. Arch Pathol Lab Med. 2013;137:756-766. [DOI] [PubMed] [Google Scholar]
- 6. Breeze AC, Jessop FA, Set PA, et al. Minimally-invasive fetal autopsy using magnetic resonance imaging and percutaneous organ biopsies: clinical value and comparison to conventional autopsy. Ultrasound Obstet Gynecol. 2011;37:317-323. [DOI] [PubMed] [Google Scholar]
- 7. Landefeld CS, Chren MM, Myers A, Geller R, Robbins S, Goldman L. Diagnostic yield of the autopsy in a university hospital and a community hospital. N Engl J Med. 1988;318:1249-1254. [DOI] [PubMed] [Google Scholar]
- 8. Ekanem VJ, Vhriterhire CO. Relevance of clinical autopsy in medical practice in Sub-Saharan Africa. Sahel Med J. 2015;18:49-56. [Google Scholar]
- 9. Royal College of Pathologists of Australasia Autopsy Working Party. The decline of the hospital autopsy: a safety and quality issue for healthcare in Australia. Med J Aust. 2004;180:281-285. [DOI] [PubMed] [Google Scholar]
- 10. Petri CN. Decrease in the frequency of autopsies in Denmark after the introduction of a new autopsy act. Qual Assur Health Care. 1993;5:315-318. [DOI] [PubMed] [Google Scholar]
- 11. Blokker BM, Weustink AC, Hunink MGM, Oosterhuis JW. Autopsy rates in the Netherlands: 35 years of decline. PLoS One. 2017;12:e0178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cox JA, Lukande RL, Nelson AM, et al. An autopsy study describing causes of death and comparing clinic-pathological findings among hospitalized patients in Kampala, Uganda. PLoS One. 2012;7:e33685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCaw-Binns A, Holder Y, Mullings J. Certification of coroners cases by pathologists would improve the completeness of death registration in Jamaica. J Clin Epidemiol. 2015;68:979-987. [DOI] [PubMed] [Google Scholar]
- 14. Blokker BM, Wagensveld IM, Weustink AC, Oosterhuis JW, Hunink MG. Non-invasive or minimally invasive autopsy compared to conventional autopsy of suspected natural deaths in adults: a systematic review. Eur Radiol. 2016;26:1159-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weustink AC, Hunink MGM, Van Dijke CF, Renken NS, Krestin GP, Oosterhuis JW. Minimally invasive autopsy: an alternative to conventional autopsy? Radiology. 2009;250:897-904. [DOI] [PubMed] [Google Scholar]
- 16. Bassat Q, Castillo P, Martınez MJ, et al. Validity of a minimally invasive autopsy tool for cause of death determination in pediatric deaths in Mozambique: an observational study. PLoS Med. 2017;14:e1002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muhe LM, McClure EM, Mekasha A, et al. A prospective study of causes of illness and death in preterm infants in Ethiopia: the SIP study protocol. Reprod Health. 2018;15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Castillo P, Ussene E, Ismail MR, et al. Pathological methods applied to the investigation of causes of death in developing countries: minimally invasive autopsy approach. PLoS One. 2015;10:e0132057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] [Google Scholar]
- 20. Muhe L, McClure EM, Nigussie A, et al. Major causes of death in preterm infants in a low resource setting: a prospective observational study in Ethiopia (The SIP study). Lancet Global Health. 2019;7:e1130–e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagendra K, Wilson C, Ravichander B, Sood S, Singh SP. Incidence and etiology of respiratory distress in newborn. Med J Armed Forces India. 1999;55:331-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghafoor T, Mahmud S, Ali S, Dogar SA. Incidence of respiratory distress syndrome. J Coll Physicians Surg Pak. 2003;13:271-273. [PubMed] [Google Scholar]
- 23. Tan GC, Hayati AR, Khong TY. Low perinatal autopsy rate in Malaysia: time for a change. Pediatr Dev Pathol. 2010;13:362-368. [DOI] [PubMed] [Google Scholar]
- 24. Ugiagbe EE, Osifo OD. Postmortem examinations on deceased neonates: a rarely utilized procedure in an African referral center. Pediatr Dev Pathol. 2012;15:1-4. [DOI] [PubMed] [Google Scholar]
- 25. Menendez C, Castillo P, Martınez MJ, et al. Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: an observational study. PLoS Med. 2017;14:e1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castillo P, Martinez MJ, Ussene E, et al. Validity of a minimally invasive autopsy for cause of death determination in adults in Mozambique: an observational study. PLoS Med. 2016;13:e1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Garg S, Punia RP, Basu S, Mohan H, Bal A. Comparison of needle autopsy with conventional autopsy in neonates. Fetal Pediatr Pathol. 2009;28(3):139-150. [DOI] [PubMed] [Google Scholar]
- 28. Celiloglu OS, Celiloglu C, Kurnaz E, Ozdemir R, Karadag A. Diagnostic contribution of postmortem needle biopsies in neonates. Turk Patoloji Derg. 2013;29:122-126. [DOI] [PubMed] [Google Scholar]
- 29. Breeze AC, Jessop FA, Whitehead AL, et al. Feasibility of percutaneous organ biopsy as part of a minimally invasive perinatal autopsy. Virchows Arch. 2008;452:201-207. [DOI] [PubMed] [Google Scholar]
- 30. Bansal MG, Punia RS, Sachdev A. Clinical and needle autopsy correlation evaluation in a tertiary care teaching hospital: a prospective study of 50 cases from the emergency department. Am J Forensic Med Pathol. 2012;33:194-196. [DOI] [PubMed] [Google Scholar]
- 31. WHO. The WHO application of ICD-10 to deaths during the perinatal period: ICD-PM. 2016. http://apps.who.int/iris/bitstream/handle/10665/249515/9789241549752-eng.pdf?sequence=1