Abstract
Background
Breast cancer is one of the most prevalent gynecologic malignancies worldwide. Despite the high sensitivity in response to chemotherapy, drug resistance occurred frequently in clinical treatment. Cryptotanshinone (CTS) is a herbal medicine and has been identified as an anti-inflammatory and anti-oxidative drug.
Methods
In vitro assays, including the cell proliferation assay, colony formation assay, Western blot analysis, transwell migration/invasion assays, and cell scratch assay were used to explore the biological activities and working mechanism of CTS. Breast cancer cells were also transfected with PKM2 expressing vectors to define the molecular mechanisms involved in CTS-mediated anti-tumor activity.
Results
We found that CTS shows anti-proliferative effects and decreases the clonogenic ability of breast cancer cells. We also found that CTS inhibited the migration and invasion activity of MCF-7 and MDA-MB-231 cells by different analyzed methods. CTS also downregulated the levels of glycolysis-related proteins, such as PKM2, LDHA, and HK2. In addition, overexpression of PKM2 recovered CTS-mediated suppression of cell proliferation, colony formation, and cell mobility of breast cancer cells. We also found PKM2 was significantly overexpressed in tumor tissues and invasive ductal breast carcinoma compared to normal tissues and patients with high PKM2 expression had worse overall survival and metastasis-free survival outcomes.
Conclusion
CTS inhibited the proliferation, migration, and invasion of breast cancer cells. The involved mechanism may refer to the downregulation of the PKM2/β-catenin axis.
Keywords: cryptotanshinone, glycolysis, breast cancer, PKM2, migration
Introduction
Breast cancer is one of the most prevalent gynecologic malignancies worldwide.1 Despite high sensitivity towards chemotherapy, drug resistance occurred frequently in clinical treatment.2 Importantly, resistance to drugs is a major obstacle in effective control of prognosis of breast cancer patients.3 Therefore, a useful agent is critical for improving the survival rate of breast cancer patients and decreasing the drug resistance.
Cryptotanshinone (CTS) is a liposoluble monomer of Tanshinones which are extracted from the dried roots and rhizomes of salvia miltiorrhiza. CTS has been identified as an anti-inflammatory and anti-oxidative drug4,5 and is demonstrated to have anti-cancer effects on various types of cancer by inhibiting cellular proliferation, migration, and invasion.6–11 Remarkably, important evidence has shown that CTS may be a potential inhibitor for glucose metabolism in ovarian cancer cells, suggesting that CTS might be a potent anticancer agent via regulating glycolysis.
Remodeling energy metabolism is the biochemical fingerprint of cancer cells,12 which is characterized by preferential reliance on glycolysis to produce energy in a non-oxygen-dependent manner,13 this phenomenon in cancer cells is called the “Warburg Effect”. Benefited by the metabolic intermediates in glycolysis, cancer cells have an advantage of survival to face the reduced nutrient supply condition, for example, increasing evidence indicates that the high glycolysis rate is essential for the resistance of chemotherapy.14,15 In other words, cancer cells rely on increased glucose consumption to escape chemotherapy and induce tumor progression. Thus, targeted glycolysis remains an attractive therapeutic strategy for cancer treatment.
In this study, we firstly defined that Cryptotanshinone suppresses glycolysis in breast cancer cells. Besides, we also adopted in vitro experiments to demonstrate that CTS inhibits migration and invasion of breast cancer cells through the PKM2/β-catenin axis. Our findings provide a new therapeutic strategy for breast cancers, and CTS might with the potential clinical significance in the development of new targeted drugs.
Materials and Methods
Cell Line and Cell Culture
The breast cancer cell lines MCF-7 and MDA-MB-231 were acquired from Shanghai Academy of Life Sciences and cultured in RPMI 1640 (Genom, China) medium and DMEM (Genom, China) medium with 10% FBS (BI, Israel). All the cells were cultured at 37°C with 5% CO2.
Cell Proliferation Assay
The effect of CTS on the viability of cells was assessed using MTT. The cells were treated with various concentrations of CTS for 24 hours after incubation in a 96-well plate. Then, MTT solution was added, incubated at 37°C for 4 hours and the sediment was dissolved in dimethyl sulfoxide (DMSO). The absorbance was measured using a micro-plate reader at 490 nm.
Colony Formation Assay
Breast cancer cells were seeded 1×103 cells in a 6-well tissue culture plate; after 24 hours they were treated with various concentrations of CTS and allowed to grow for 6 days (MCF-7) or 10 days (MDA-MB-231) to form colonies. Cells were stained by 0.1% crystal violet. The total number of colonies formed on each well were analyzed.
Cell Scratch Assay
The cells were seeded into 6-well plates and a scratch wound was created by a P200 pipette tip. After 24 hours, fresh medium without FBS containing either CTS or shikonin was added. Cell migration was observed under an inverted microscope. The percentage of wound closure was measured, using the wound areas from time zero (T0) and 24 hours (T24) by the following formula: Wound closure (%) = (T0–T24)/T0.
Transwell Migration/Invasion Assay
For the migration assay, 5×104 cells in 200 µL of the serum-free medium were seeded in the 8-mm poresize the upper compartment of the chamber in 24-well cell culture insert companion plates. Then, 600 µL of complete medium containing 10% FBS was added into the lower chamber. After incubation for another 12 hours, the chambers were removed and the cells on the upper surface of membrane were wiped off with cotton swabs, and the migrated cells on the lower surface were stained with 0.5% crystal violet for 30 minutes. Finally, the cells were observed with an inverted microscope, and images were obtained. For the invasion assay, 1×105 of the cells were seeded to the upper surface of polycarbonate membranes and covered with the 50 µL Matrigel (Becton-Dickinson) layer. The invasion assay was incubated for another 48 hours and subsequent procedures were similar to the migration assay.
Western Blot Analysis
Protein was extracted from MCF-7 and MDA-MB-231, and concentration calibration protein loading was measured using the BCA kit (Beyotime, China). The proteins were loaded on 10% SDS-PAGE gel and transferred onto polyvinylidene fluoride (PVDF) membranes. Therein, 20 μg of protein mixed with SDS loading buffer was loaded per lane. We used 5% nonfat milk to block membranes, washed with TBST, and incubated with different antibodies overnight at 4°C. Then, followed by incubating with a secondary antibody for 2 hours, the membranes were visualized with an ECL-plus detection system.
PKM2 Plasmid Transfection
The PKM2 overexpression plasmid was purchased from Addgene, Inc. (Cambridge, MA, USA). 1×105 MCF-7 cancer cells and MDA-MB-231 cancer cells were seeded in 6-well cell culture dishes and transfected the PKM2 expressing vector using the TransIT-X2 transfection reagent (Mirus, Madison, WI, USA) according to the manufacturer’s instructions. Further analysis was performed after 48 hours of transfection.
Statistical Analysis
Data are presented as mean±standard deviation (SD), and statistical differences between treatment groups were analyzed using Students’ t-test by GraphPad Prism 5.0. Significant differences were considered as P<0.05.
Results
CTS Inhibits Proliferation of Breast Cancer Cells
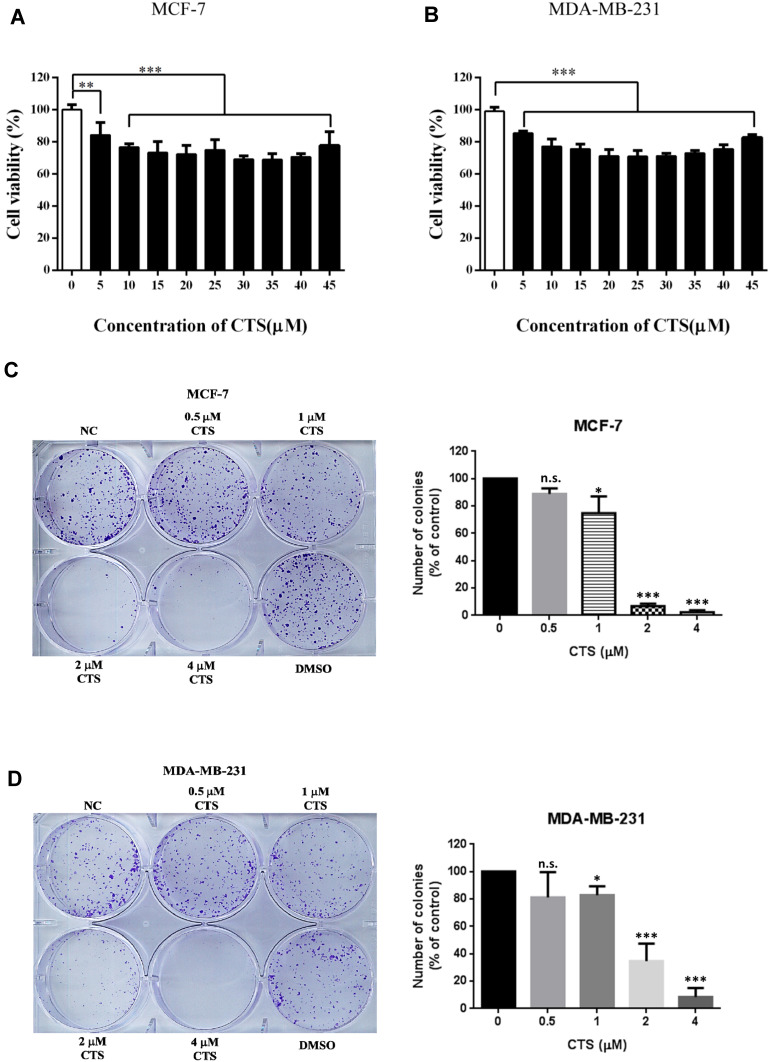
An ideal anticancer drug must be fewer cytotoxic effects to normal cells. Some reported showed that CTS had cytotoxic effects in cancer cells but had a slight effect on normal cells.16,17 To study whether CTS causes toxicity on breast cancer cells, the cell viability was tested at various concentrations of CTS in MCF-7 cells and MDA-MB-231 by an MTT assay. The results showed that the concentration of CTS had obviously influenced cell survival on both cells (Figure 1A and B) (P<0.05). After 24 hours of incubation, treatment with various dosages of CTS decreased the number of viable cells. In colony formation assay, we also found that treatment with CTS significantly decreased the clonogenic survival rate in a dose-dependent manner in both cells (Figure 1C and D). These results suggest that CTS significantly inhibits cell proliferation and survival of breast cancer cells.
Figure 1.
CTS decreases cell proliferation. (A and B) After the cells were treated with different concentration of CTS for 24 hours, cell proliferation was evaluated by MTT assay. (C and D) Colony formation assay of cells treated with different concentrations of CTS. Data are presented as mean±SD (n=6). *Represented P<0.05, ***Represented P<0.001, compared with the blank control group (NC).
CTS Inhibits Migration and Invasion of Breast Cancer Cells
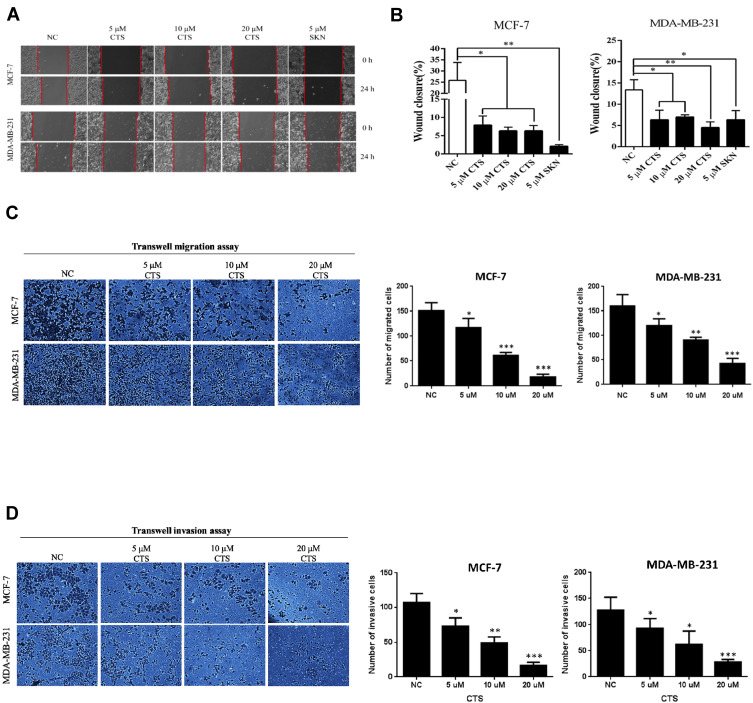
Breast cancer has high invasiveness and high recurrence and cancer metastasis is the leading cause of death from advanced breast cancer.18 Therefore, we used cell scratch assay to evaluate the in vitro migration ability of CTS on breast cancer cells (Figure 2A). We found that the migration ability of MCF-7 and MDA-MB-231 cells was significantly decreased after 24 hours intervention with SKN and CTS, and the difference was statistically significant (P<0.05) (Figure 2B). Following, we used the transwell migration assay to further define whether the migratory ability significantly decreased by CTS. Consistently, we found CTS decreased cell migratory ability in a dose-dependent manner by Transwell migration assay (Figure 2C). Furthermore, we evaluated the effects of CTS on cell invasion of MCF-7 and MDA-MB-231 cells. After treatment with CTS, the invasive abilities of MCF-7 and MDA-MB-231 cells were significantly decreased compared to the untreated groups (Figure 2D). These data further indicated that CTS inhibits migration and the invasion ability of breast cancer cell lines.
Figure 2.
CTS suppresses the migration and invasion of breast cancer cells. (A and B) The migration ability of breast cancer cells was inhibited by shikonin (SKN) and CTS in the cell scratch assay. Shikonin (SKN) acts as the positive control for inhibition of migration ability. (C) Transwell migration assays were detected after treated with different concentrations of CTS in MCF-7 and MDA-MB-231 cells. (D) Transwell invasion assays were detected after treated with different concentrations of CTS in MCF-7 and MDA-MB-231 cells. Data are presented as mean±SD (n=3). *Represented P<0.05, **Represented P<0.01, ***Represented P<0.001, compared with the blank control group (NC).
CTS Inhibits Glycolysis and PKM2/β-Catenin Signaling of Breast Cancer Cells
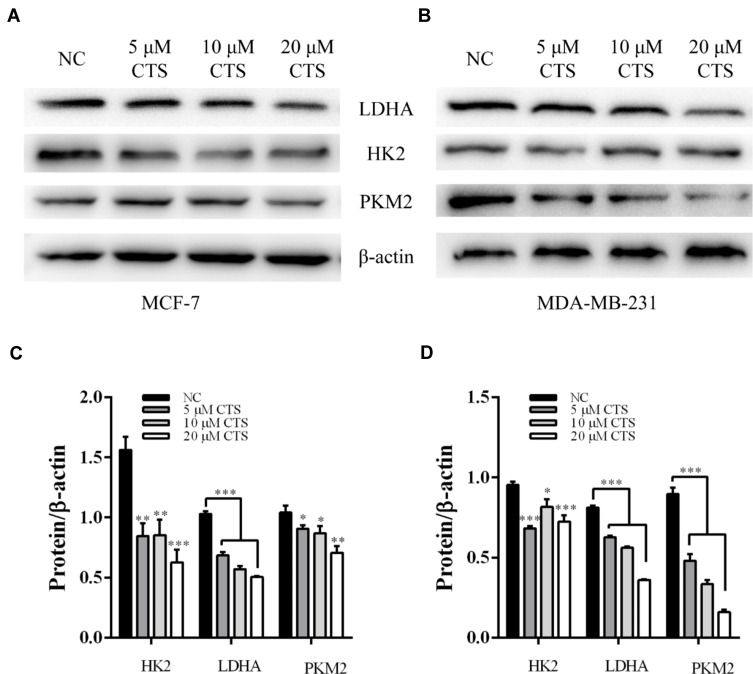
The cancer cell produces additional energy by increased glycolysis activity to promote tumorigenesis and breast cancer progression.19 Referring to published literature, glycolysis related genes were high expression in breast cancer tissues compared with normal tissue.20 Therefore, we examined whether CTS regulates glycolysis related protein LDHA, HK2, and PKM2 expression in breast cancer cell lines. As shown in Figure 3, the Western blot results indicated that CTS (5, 10, 20 μM) strongly decreased the expression of LDHA, HK2, PKM2 in protein levels of both breast cancer cell lines. Together, these data showed that CTS significantly regulated breast cancer cells’ glycolysis.
Figure 3.
CTS inhibits glycolysis in MCF-7 and MDA-MB-231 cells. (A and C) In MCF-7 cell, comparison of PKM2, GLUT1, LDHA, and HK2 expression level among groups; (B and D) In MDA-MB-231 cell, comparison of PKM2, GLUT1, LDHA, and HK2 expression level among groups; Data are presented as mean±SD (n=3). *Represented P<0.05, **Represented P<0.01, ***Represented P<0.001, compared with the blank control group (NC).
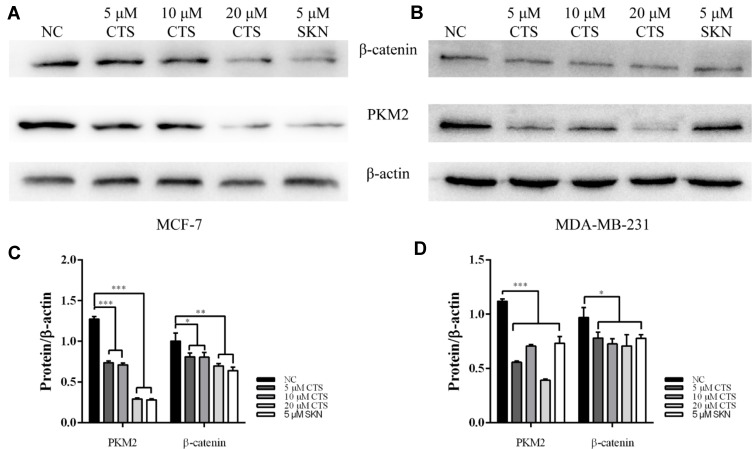
PKM2 has been shown to be involved in the maintenance of cancer stem cell-like phenotypes, angiogenesis.21,22 As an important regulator, PKM2 can mediate β-catenin to promote EMT.23 It has been found earlier that CTS can affect the expression of PKM2.24,25 Therefore, we subsequently performed Western blotting assay to define the effects of CTS on the PKM2/β-catenin signaling pathway. After treatment with SKN and different concentrations of CTS for 24 hours, we found that the expressions of PKM2 and β-catenin in MCF-7 and MDA-MB-231 cells were decreased significantly (Figure 4).
Figure 4.
CTS downregulates PKM2/β-catenin axis. (A and C) in MCF cell and (B and D) in the expression levels of PKM2 and β-catenin in breast cancer cells were decreased by shikonin (SKN) and CTS. *Represented P<0.05, **Represented P<0.01, ***Represented P<0.001, compared with the blank control group (NC).
PKM2 Regulates CTS Sensitivity in Breast Cancer Cells
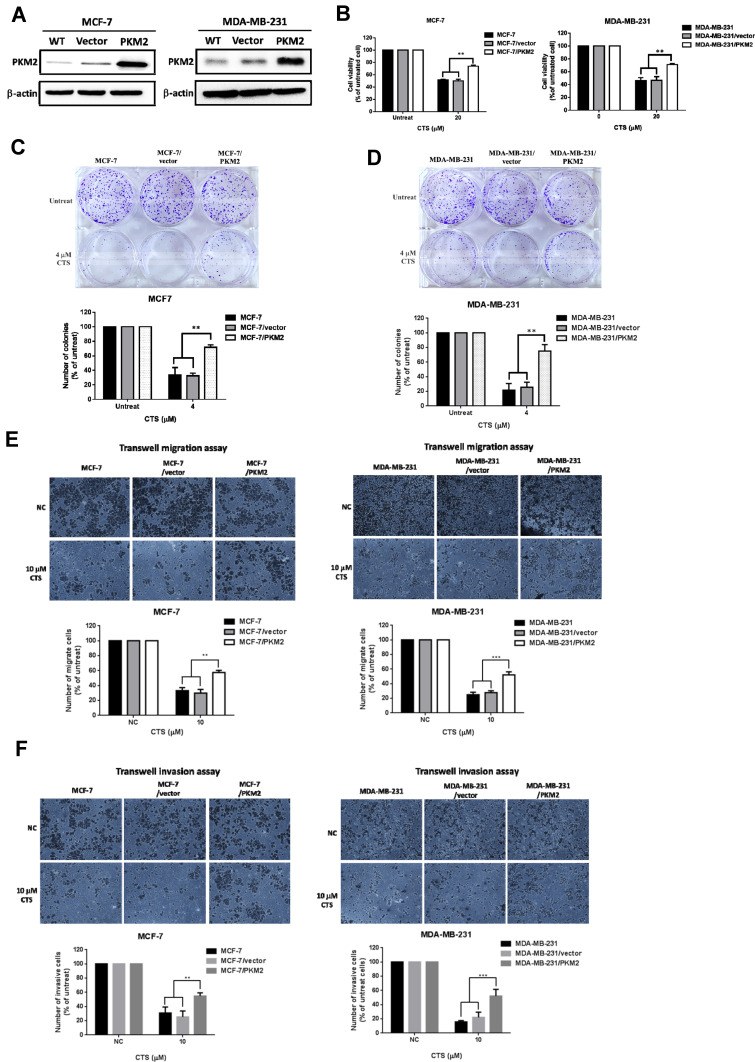
To define whether PKM2 play an important functional role in CTS-mediated anti-cancer activities of breast cancer cells, we overexpressed PKM2 plasmid in MCF-7 and MDA-MB-231 cells. As shown in Figure 5A, overexpression of PKM2 successfully increased PKM2 expression in MCF-7 and MDA-MB-231 cells. Next, we used MTT assay to investigate whether PKM2 affects CTS sensitivity in breast cancer cell lines. We found that overexpression of PKM2 in MCF-7 and MDA-MB-231 cells led to reduced CTS sensitivity (Figure 5B). Consistently, in the colony formation assay, the results have been shown that not only CTS-suppressed colony formation ability (Figure 5C and D) but also the migration/invasion activity (Figure 5E and F) were significantly recovered by overexpression of PKM2, as compared to control cells. These results indicated that PKM2 mediated the suppressive effects of CTS on cell proliferation, colony formation, and cell mobility of breast cancer cells.
Figure 5.
Overexpression of PKM2 abolished CTS-induced cell death and cell mobility suppression of breast cancer cells. (A) Transfection of PKM2 plasmids in MCF-7 and MDA-MB-231 cells and analyzed the expression of PKM2 in the indicated cells by Western blot. (B) Overexpression of PKM2 decreased the CTS-mediated suppression of cell survival ability. Cell survival ability of indicated groups were analyzed by MTT assay. Overexpression of PKM2 in MCF-7 cells (C) and MDA-MB-231 cells (D) significantly increased colony forming ability of cells treated with CTS by colony formation assay. (E) The effects of CTS on cell migration were analyzed using Transwell migration assay in MCF-7 and MDA-MB-231 cells stably expressing PKM2. (F) Overexpression of PKM2 reversed the effect of CTS on cell invasion. Data are presented as mean±SD (n=3). **Represented P<0.01, ***Represented P<0.001, compared with the blank control group (NC).
PKM2 is a Poor Prognostic Biomarker and Associated with Breast Cancer Risk
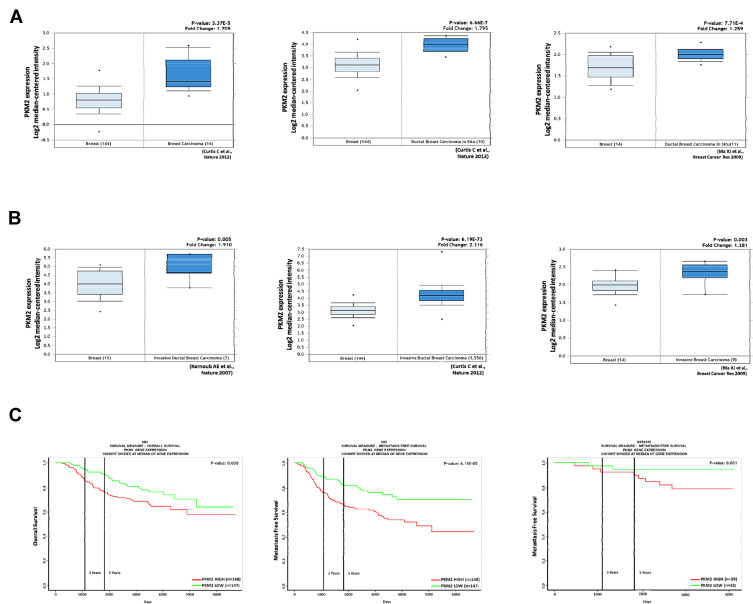
Some recent studies showed that PKM2 regulates cell growth, migration, invasion, and cancer progression in breast cancer.26–29 We found that PKM2 was overexpressed in breast cancer tissues compared to normal tissues by the Oncomine database (Figure 6A). Among them, we noted that PKM2 was also higher in invasive ductal breast carcinoma (Figure 6B). In addition, we observed that patients with high PKM2 expression had worse overall survival and metastasis-free survival outcomes in the PROGgeneV2 online database (http://genomics.jefferson.edu/proggene/index.php) (Figure 6C). These results suggest that the expression of PKM2 was significantly associated with poor survival outcomes in breast cancer patients.
Figure 6.
Expression of PKM2 positively associated with poor prognosis of breast cancer patients. (A) PKM2 expression positively correlates with breast cancer tissue and normal tissue analyzed by the Oncomine database. (B) PKM2 expression positively correlates with invasive breast cancer tissue and breast cancer tissue in the Oncomine database. The fold change (Log2 median-centered intensity) and P-value are shown within each box plot. (C) Analysis of overall survival and metastasis-free survival in breast cancer patients. High PKM2 expression correlates with poor survival of breast cancer patients.
Discussion
Compounds isolated from herbal medicine are widely used in cancer treatment.30 Cryptotanshinone (CTS), extracted from dried roots of salvia miltiorrhiza, have widespread applications as anti-inflammatories and anti-oxidants.31 In this study, we demonstrated the anti-tumor and bioactivity of CTS on breast cancer cells.
CTS has been reported to inhibit development of different cancers. CTS showed the anti-tumor and chemosensitization effects on ovarian cancer cells in vitro.6 It also suppressed migration and invasion of human melanoma cancer cells, and induced apoptosis via the ROS-mitochondrial apoptotic pathway.32 In this study, we found that CTS significantly inhibited the proliferation of breast cancer cells, and reduced expression of glycolysis-related proteins. We also observed that migration and invasion ability of breast cancer cells significantly suppressed by CTS via PKM2/β-catenin axis.
Glycolysis is an ATP-supporter in human cells, especially under low-oxygen environment. In cancer cells, glycolysis is considered as a major way to support cancer cells growth, and facilitate energy production, invasion, and redox equilibrium.33 Enhanced glycolysis has been observed in human tumor tissues.34,35 It is noteworthy that several enzymes have been shown to influence glycolysis in breast cancer cells, especially HK2, LDHA, and PKM2.12,36,37 Inhibition of LDHA reduced the pro-survival autophagy and drug resistance in MCF-7 cells.38 More recently, HK2 has been considered as an important factor in tumorigenesis in mouse models and breast cancer cells, both in vitro and in vivo.39 PKM has been shown to significantly suppress NF-κB signaling and NF-κB target genes, knockdown of PKM effectively inhibits cell growth and migration.27
Modulation of PKM2 expression could promote cytotoxic autophagy and apoptosis in breast cancer cells.40 Our data indicated that breast cancer cells treated with CTS displayed a low proliferation rate and different concentrations of CTS significantly inhibit the cell migration and invasion of breast cancer cells. Moreover, we found that CTS could inhibit the expressing levels of glycolysis-related proteins, such as PKM2, LDHA, and HK2.
PKM2, a rate-limiting enzyme in glycolysis, has been widely reported to play a role in different processes of cancer development. In recent years, PKM2 has been found to be an important factor in various biological functions of cancer cells, including regulation of cell cycle, resistance to chemotherapy, and cancer metastasis.41,42 PKM2 exists in two forms, tetramer and dimer. When PKM2 was phosphorylated at serine and tyrosine sites, it's molecular structure changed from tetramer to dimer.43 The tetramer form of PKM2 has the strongest activity to promote the complete oxidative decomposition of glucose to generate ATP through oxidative phosphorylation. Therefore, formation of PKM2 tetramer could inhibit the occurrence and development of tumors.44 The inactive dimer form of PKM2 promoted the Warburg effect, or aerobic digestion of glucose. In tumor cells, the dimer form of PKM2 is dominant, which provides precursors for biosynthesis and provides a metabolic advantage for tumor cells. In addition to its role as a pyruvate kinase, PKM2 is a transcriptional coactivator of protein kinases and genes to mediate cell proliferation, migration, and apoptosis. Wnt/β-catenin signaling plays essential roles in diseases development in humans, including cancer and myocardial infarction.45 Some reports pointed out that PKM2 is translocated into the nucleus in cancer cells as a transcriptional factor, and nuclear PKM2 regulates β-catenin transactivation.46 Notably, β-catenin mediates many important functions of cancer cells, such as cell migration, invasion, and angiogenesis. A clinical study concludes that the expression of β-catenin in TNBC tissue might be closely correlated with survival prognosis.47 In our study, we also found that CTS effectively suppressed breast cancer migration and invasion, the underlying mechanism may refer to the downregulation of PKM2/β-catenin axis and overexpression of PKM2 decreased CTS sensitivity in breast cancer cells.
Conclusion
In conclusion, we have demonstrated that CTS exerts an anti-cancer effect of breast cancer cells by suppressing cell proliferation, migration, and invasion of breast cancer cells in vitro. In addition, CTS could markedly reduce the expression of glycolysis-related proteins and PKM2/β-catenin signaling in breast cancer cells. Overexpression of PKM2 recovered CTS-mediated suppression of cell proliferation, colony formation, and cell mobility of breast cancer cells.
Funding Statement
This work was supported by the Ministry of Science and Technology grants from Taiwan (104-2320-B-038-053-MY3), (108-2745-8-038-002-), (108-2320-B-038-020-), (108-2314-B-038-121-) and (109-2314-B-038-121-).
Abbreviations
BC, breast cancer; CTS, cryptotanshinone.
Disclosure
Jiefeng Zhou, Shicong Du, and Haoran Wu are employees of Ningbo AJcore Biosciences Inc. Chang-Wei Li is an employee of Ningbo AllBiolife Biotech Inc. The authors report no other potential conflicts of interest in this work.
References
- 1.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203 [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Wang Y, Zhong J. Side population cells and drug resistance in breast cancer. Mol Med Rep. 2015;11(6):4297–4302. doi: 10.3892/mmr.2015.3291 [DOI] [PubMed] [Google Scholar]
- 3.Merikhian P, Ghadirian R, Farahmand L, Mansouri S, Majidzadeh-A K. MUC1 induces tamoxifen resistance in estrogen receptor-positive breast cancer. Expert Rev Anticancer Ther. 2017;17(7):607–613. doi: 10.1080/14737140.2017.1340837 [DOI] [PubMed] [Google Scholar]
- 4.Feng Z, Zheng W, Li X, et al. Cryptotanshinone protects against IL-1β-induced inflammation in human osteoarthritis chondrocytes and ameliorates the progression of osteoarthritis in mice. Int Immunopharmacol. 2017;50:161–167. doi: 10.1016/j.intimp.2017.06.017 [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Wang X, Zhang X, Liang C. Cryptotanshinone attenuates oxidative stress and inflammation through the regulation of Nrf‐2 and NF‐κB in mice with unilateral ureteral obstruction. Basic Clin Pharmacol Toxicol. 2018;123(6):714–720. doi: 10.1111/bcpt.13091 [DOI] [PubMed] [Google Scholar]
- 6.Jiang G, Liu J, Ren B, et al. Anti-tumor and chemosensitization effects of Cryptotanshinone extracted from Salvia miltiorrhiza Bge. on ovarian cancer cells in vitro. J Ethnopharmacol. 2017;205:33–40. doi: 10.1016/j.jep.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 7.Pan Y, Shi J, Ni W, et al. Cryptotanshinone inhibition of mammalian target of rapamycin pathway is dependent on oestrogen receptor alpha in breast cancer. J Cell Mol Med. 2017;21(9):2129–2139. doi: 10.1111/jcmm.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen L, Zhang G, Lou Z, Xu G, Zhang G. Cryptotanshinone enhances the effect of Arsenic trioxide in treating liver cancer cell by inducing apoptosis through downregulating phosphorylated-STAT3 in vitro and in vivo. BMC Complement Altern Med. 2017;17(1):106. doi: 10.1186/s12906-016-1548-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu D, Lin T-H, Li S, et al. Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett. 2012;316(1):11–22. doi: 10.1016/j.canlet.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Wen G, Sun L, et al. Cryptotanshinone inhibits cellular proliferation of human lung cancer cells through downregulation of IGF-1R/PI3K/Akt signaling pathway. Oncol Rep. 2018;40(5):2926–2934. doi: 10.3892/or.2018.6638 [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Chen C, Duanmu J, et al. Cryptotanshinone inhibits the growth and invasion of colon cancer by suppressing inflammation and tumor angiogenesis through modulating MMP/TIMP system, PI3K/Akt/mTOR signaling and HIF-1α nuclear translocation. Int Immunopharmacol. 2018;65:429–437. doi: 10.1016/j.intimp.2018.10.035 [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Wu K, Liu Y, Shi L, Tao K, Wang G. Prognostic significance of metabolic enzyme pyruvate kinase M2 in breast cancer: a meta-analysis. Medicine. 2017;96(46). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanciulli M, Bruno T, Giovannelli A, et al. Energy metabolism of human LoVo colon carcinoma cells: correlation to drug resistance and influence of lonidamine. Clin Cancer Res. 2000;6(4):1590–1597. [PubMed] [Google Scholar]
- 15.Lu C-W, Lin S-C, Chen K-F, Lai -Y-Y, Tsai S-J. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem. 2008;283(42):28106–28114. doi: 10.1074/jbc.M803508200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Yu W, Cai G, et al. A new synthetic derivative of cryptotanshinone KYZ3 as STAT3 inhibitor for triple-negative breast cancer therapy. Cell Death Dis. 2018;9(11):1098. doi: 10.1038/s41419-018-1139-z [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Liu C, Sun HN, Luo YH, et al. Cryptotanshinone induces ROS-mediated apoptosis in human gastric cancer cells. Oncotarget. 2017;8(70):115398–115412. doi: 10.18632/oncotarget.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274(2):113–126. doi: 10.1111/joim.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phan LM, Yeung SC, Lee MH. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol Med. 2014;11(1):1–19. doi: 10.7497/j.issn.2095-3941.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W, Xu M, Li Y, et al. Comprehensive analysis of the association between tumor glycolysis and immune/inflammation function in breast cancer. J Transl Med. 2020;18(1):92. doi: 10.1186/s12967-020-02267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed N, Escalona R, Leung D, Chan E, Kannourakis G. Tumour microenvironment and metabolic plasticity in cancer and cancer stem cells: perspectives on metabolic and immune regulatory signatures in chemoresistant ovarian cancer stem cells. Semin Cancer Biol. 2018;53:265–281. doi: 10.1016/j.semcancer.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 22.Snyder V, Reed-Newman TC, Arnold L, Thomas SM, Anant S. Cancer stem cell metabolism and potential therapeutic targets. Front Oncol. 2018;8:203. doi: 10.3389/fonc.2018.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabakov A, Yakimova A, Matchuk O. Molecular chaperones in cancer stem cells: determinants of stemness and potential targets for antitumor therapy. Cells. 2020;9(4):892. doi: 10.3390/cells9040892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y, Zheng Q, Ni W, et al. Breaking glucose transporter 1/Pyruvate Kinase M2 glycolytic loop is required for cantharidin inhibition of metastasis in highly metastatic breast cancer. Front Pharmacol. 2019;10:590. doi: 10.3389/fphar.2019.00590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi P, Li Y, Liu X, et al. Cryptotanshinone suppresses non-small cell lung cancer via microRNA-146a-5p/EGFR axis. Int J Biol Sci. 2019;15(5):1072–1079. doi: 10.7150/ijbs.31277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He X, Du S, Lei T, et al. PKM2 in carcinogenesis and oncotherapy. Oncotarget. 2017;8(66):110656–110670. doi: 10.18632/oncotarget.22529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma C, Zu X, Liu K, et al. Knockdown of pyruvate kinase M inhibits cell growth and migration by reducing NF-kB activity in triple-negative breast cancer cells. Mol Cells. 2019;42(9):628–636. doi: 10.14348/molcells.2019.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zahra K, Dey T, Ashish MSP, Pandey U. Pyruvate kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol. 2020;10:159. doi: 10.3389/fonc.2020.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Q, Luo S, Tan Q, et al. The role of pyruvate kinase M2 in anticancer therapeutic treatments. Oncol Lett. 2019;18(6):5663–5672. doi: 10.3892/ol.2019.10948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han B, Huang H, Li Z, et al. Therapeutic effects of chinese medicine herb pair, huzhang and guizhi, on monosodium urate crystal-induced gouty arthritis in rats revealed by anti-inflammatory assessments and NMR-based metabonomics. Evid Based Complement Alternat Med. 2016;2016:1–12. doi: 10.1155/2016/9398435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang S, Wen Q, Liu P, et al. Effects of cryptotanshinone on the expression levels of inflammatory factors in myocardial cells caused by Ang II and its mechanism. Int J Clin Exp Med. 2015;8(8):12617. [PMC free article] [PubMed] [Google Scholar]
- 32.Ye T, Zhu S, Zhu Y, et al. Cryptotanshinone induces melanoma cancer cells apoptosis via ROS-mitochondrial apoptotic pathway and impairs cell migration and invasion. Biomed Pharmacother. 2016;82:319–326. doi: 10.1016/j.biopha.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 33.Qureshi AS, Ali S. Review: warburg effect and renal cancer caused by errs in fumarate hydratase encoding gene. Pak J Pharm Sci. 2019;32(2):743–749. [PubMed] [Google Scholar]
- 34.Brauer HA, Makowski L, Hoadley KA, et al. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res. 2013;19(3):571–585. doi: 10.1158/1078-0432.CCR-12-2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budczies J, Denkert C, Müller BM, et al. Remodeling of central metabolism in invasive breast cancer compared to normal breast tissue–a GC-TOFMS based metabolomics study. BMC Genomics. 2012;13(1):334. doi: 10.1186/1471-2164-13-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown RS, Goodman TM, Zasadny KR, Greenson JK, Wahl RL. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol. 2002;29(4):443–453. doi: 10.1016/S0969-8051(02)00288-3 [DOI] [PubMed] [Google Scholar]
- 37.Huang X, Li X, Xie X, et al. High expressions of LDHA and AMPK as prognostic biomarkers for breast cancer. Breast. 2016;30:39–46. doi: 10.1016/j.breast.2016.08.014 [DOI] [PubMed] [Google Scholar]
- 38.Das CK, Parekh A, Parida PK, Bhutia SK, Mandal M. Lactate dehydrogenase A regulates autophagy and tamoxifen resistance in breast cancer. Biochim Biophys Acta Mol Cell Res. 2019;1866(6):1004–1018. doi: 10.1016/j.bbamcr.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 39.Patra KC, Wang Q, Bhaskar PT, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–228. doi: 10.1016/j.ccr.2013.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewinska A, Adamczyk-Grochala J, Kwasniewicz E, Deregowska A, Wnuk M. Ursolic acid-mediated changes in glycolytic pathway promote cytotoxic autophagy and apoptosis in phenotypically different breast cancer cells. Apoptosis. 2017;22(6):800–815. doi: 10.1007/s10495-017-1353-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi H, Li D, Zhang J, et al. Silencing of pkm2 increases the efficacy of docetaxel in human lung cancer xenografts in mice. Cancer Sci. 2010;101(6):1447–1453. doi: 10.1111/j.1349-7006.2010.01562.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tung S, Shi Y, Wong K, et al. PPARα and fatty acid oxidation mediate glucocorticoid resistance in chronic lymphocytic leukemia. Blood. 2013;122(6):969–980. doi: 10.1182/blood-2013-03-489468 [DOI] [PubMed] [Google Scholar]
- 43.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43(7):969–980. doi: 10.1016/j.biocel.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 44.Anastasiou D, Yu Y, Israelsen WJ, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8(10):839. doi: 10.1038/nchembio.1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim W, Kim M, Jho E-H. Wnt/β-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450(1):9–21. doi: 10.1042/BJ20121284 [DOI] [PubMed] [Google Scholar]
- 46.Yang W, Xia Y, Ji H, et al. Nuclear PKM2 regulates β-catenin transactivation upon EGFR activation. Nature. 2011;480(7375):118. doi: 10.1038/nature10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J, Li M, Chen D, et al. Expression of C-myc and β-catenin and their correlation in triple negative breast cancer. Minerva Med. 2017;108(6):513–517. doi: 10.23736/S0026-4806.17.05213-2 [DOI] [PubMed] [Google Scholar]