Abstract
Introduction
Breast cancer is a serious threat to human health. It is meaningful to study the pathogenesis of breast cancer. lncRNAs have been found to play vital roles in numerous biological processes including development, immunology and cancer.
Methods
qRT-PCR was performed to examine the expressions of PART1 and miR-4516. CCK-8 assay, colony formation assay and transwell assay were used to examine the progression of breast cancer cells.
Results
In this study, we showed that lncRNA PART1 was highly expressed in breast cancer cells. Knockdown of PART1 induced decreased proliferation, invasion and migration of breast cancer cells. Moreover, we found that PART1 can bind to miR-4516 directly. We also found that inhibition of miR-4516 could rescue the decreased proliferation, migration and invasion of breast cancer cells induced by knockdown of PART1.
Discussion
lncRNA PART1 and miR-4516 were proven to be involved in the progression of many cancers. However, the roles of lncRNA PART1 and miR-4516 in the regulation of breast cancer remain unknown. Here, we demonstrated that PART1 can bind to miR-4516 to decrease the expression of miR-4516 and promote the development of breast cancer.
Keywords: PART1, miR-4516, breast cancer, proliferation, migration, invasion
Introduction
Breast cancer is a malignant tumor that occurs in breast epithelial tissue.1 Ninety-nine percent of breast cancers occur in women while only 1% of breast cancers occur in men.2 In situ breast cancer is not fatal. However, breast cancer cells will lose the connection between cells and easy to fall off. Once cancer cells fall off, they will metastasize to the whole body through the blood or lymph nodes.3 In recent years, the incidence of breast cancer is increasing.4–6 However, the pathogenesis of breast cancer remains unclear.7
Accumulating evidence have shown that lncRNAs exert important roles in the progression of breast cancer. lncRNAs are newly identified RNAs that have over 200 nucleotides.8,9 Most lncRNAs have no or little protein-coding potential.10 Evidence have implied that lncRNAs are important regulators in breast cancer.11 For instance, lncRNA ANCR can impair the invasion and metastasis of breast cancer through degradation of EZH2.12 lncRNA PVT1 is upregulated by SOX2 and mediates the cell proliferation and invasion of breast cancer.13 However, the role of lncRNA PART1 in the regulation of breast cancer remains unclear.
miRNAs are a kind of small non-coding RNAs that negatively regulate the mRNA translation at the post-transcriptional level.14,15 Many kinds of research have demonstrated that lncRNAs often target miRNAs directly to exert their role in promoting the progression of cancer.16 miR-4516 has been proven to regulate the progression of many cancers, including glioblastoma, hepatocellular carcinoma, papillary thyroid carcinomas and so on.17–19 However, the role of miR-4516 in the regulation of breast cancer remains unclear.
Materials and Methods
Samples and Cell Lines
Human breast cancer samples and adjacent healthy breast tissues were obtained from 31 breast cancer patients (male, n=31; age range, 46–73 years; years, 2016–2019) under surgery at The First People’s Hospital of Jingzhou. All samples were confirmed as breast cancer by postoperative histopathological examination and were kept in liquid nitrogen before use. All procedures were performed in accordance with the Helsinki Declaration. Exclusion criteria included radiotherapy or chemotherapy prior to surgical treatment, a prior history of cancer, and a lack of the written informed consent. This study was approved by the Ethics Committee of The First People’s Hospital of Jingzhou. All written informed consents were received from patients.
Human breast cancer cell lines: MCF-7, SKBR3, BT-20, MDA-MB-231, ZR-75-1 and one normal breast cell line: MCF-10A was obtained from American Type Culture Collection (ATCC, USA). MCF7, SKBR3 and MDA-MB-231 were cultured in DMEM medium supplemented with 100 mg/mL streptomycin, 100 U/mL penicillin and 10% FBS; BT20 cells and ZR-75-1 cells were cultured in RPMI-1640 medium supplemented with 100 mg/mL streptomycin, 100 U/mL penicillin and 10% FBS. MCF-10A cells were cultured in M-171 medium supplemented with mammary epithelial growth factors (Invitrogen/Life Technologies, USA).20 All cells were cultured at 37°C in 5% CO2.
Plasmids and Transfection
Control, miR-4516 mimic, miR-4516 inhibitor, shRNA specifically targeting PART1, and scrambled negative control shRNA were chemically synthesized by GenePharma Co. Transfection assays were performed for transient expression as previously described.21 miR-4516 mimic: 5ʹ-GGGAGAAGGGUCGGGGC-3ʹ; miR-4516 inhibitor: 5ʹ-CCCUCUUCCCAGCCCG-3ʹ; miRNA control: 5ʹ-UAAGGCUAUGAAGAGAUAC-3ʹ; shPART1-1: 5ʹ-GAAAACGCAGCTACACCTGG-3ʹ; shPART1-2: 5ʹ-GACTACATATGCATTAAGG-3ʹ; Control shRNA: 5ʹ-AAGGCUAUGAAGAGAUAC-3ʹ. Breast cancer cells were transfected with 100 nM miR-4516 mimics, miR-control or miR-4516 inhibitors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
pMIR-PART1-WT or pMIR-PART1-Mutant and miR-4516 mimics were co-transfected into MCF-7 cells together with pRL-CMV Renilla luciferase reporter. After 48h, data was measured using luciferase assay kit (Promega, Madison, WI, USA). Firefly luciferase activity was normalized against Renilla luciferase activity.21
Quantitative Reverse Transcription-PCR (qRT-PCR)
Total RNA was isolated by Trizol reagent (Invitrogen, Carlsbad, CA, USA) from indicated cells according to the protocol of the manufacturer. And, 1μg total RNA was reverse-transcribed into cDNAs according to the manufacturer’s instructions using the PrimeScript RT reagent Kit (Promega, Madison, WI, USA). Quantitative reverse transcription PCR was performed using SYBR Green PCR Master Mix reagents (Takara) in 7300 Real-Time PCR System (Applied Biosystems). The primers were listed as below:
hsa-miR-4516 Forward primer 5ʹ-CACTCCAGCTGGGGGGAGAAGGG-3ʹ
hsa-miR-4516 Reverse primer 5ʹ-CTCAACTGGTGTCGTGGAGTCG-3ʹ
lncRNA PART1 Forward primer 5ʹ-GGACTCGTGCTTCTCGTACGCTGG-3ʹ
lncRNA PART1 Reverse primer 5ʹ- GCCTGCCCTTTGGTTTCTGGGAC-3ʹ
18S Forward, 5ʹ-GTAACCCGTTGAACCCCATT-3ʹ;
18S Reverse, 5ʹ-CCATCCAATCGGTAGTAGCG-3ʹ;
Cell Counting Kit-8 Assays
MCF-7 cells and BT-20 cells were seeded in 96-well plates and cultured at the DMEM medium with 100 mg/mL streptomycin, 100 U/mL penicillin and 10% FBS at 37°C containing 5% CO2. The proliferation abilities of MCF-7 cells and MDA-MB-231 cells were examined at 24, 48, and 72 hours after transfection according to the manufacturer’s protocols using Cell Counting Kit-8 CCK-8 (7 sea biotech, Shanghai, China).
Transwell Assay
Invasion or migration chamber was pre-coated by Matrigel (BD Biosciences, Cowley, United Kingdom) for transwell assay. 4×104 MCF-7 cells or BT-20 cells were seeded into the upper chamber with serum-free DMEM medium. And, the lower chamber was supplemented with 500 μL DMEM containing 10% FBS. After 24 hours of incubation, cells in the lower chamber were fixed by 4% paraformaldehyde and stained by 0.1% crystal violet for 30 min.
Statistical Analysis
All results were analyzed using GraphPad Prism 6 software. And, all results were expressed as mean ± SD. A Student’s t-test was used to analyze the differences between the two groups. A one-way ANOVA followed by a Tukey’s post hoc test was used for multiple comparisons. P < 0.05 was considered to be significant.
Results
lncRNA PART1 Was Highly Expressed in Breast Cancer Cells
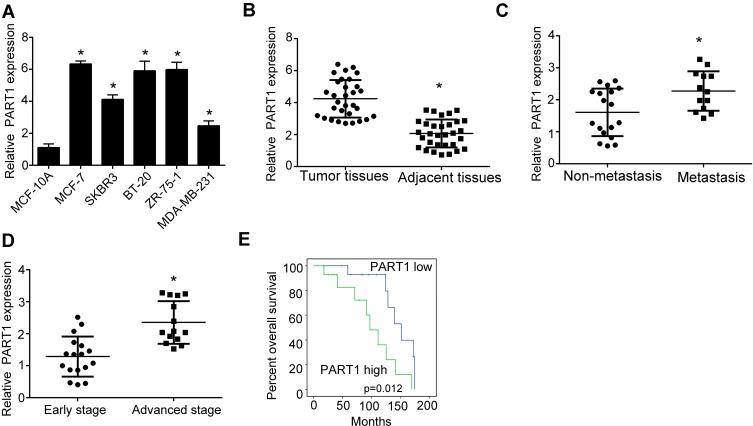
To explore the role of lncRNA PART1 in the regulation of breast cancer, we firstly examined the expression of lncRNA PART1 in breast cancer cell lines. Through qRT-PCR assay, we found that lncRNA PART1 was highly expressed in breast cancer cell lines, MCF-7, SKBR3, BT-20, MDA-MB-231, ZR-75-1 compared to normal breast cell line, MCF-10A (Figure 1A). Moreover, we also examined the expression of PART1 in human breast cancer samples. Consistently, we found that the expression of PART1 in breast cancer samples was relatively higher than normal tissues (Figure 1B), which indicates that PART1 may involve in the regulation of breast cancer. To explore if PART1 was associated with the metastasis or advanced stage of breast cancer, we performed qRT-PCR and found the higher expression of PART1 in metastasis and advanced stage samples (Figure 1C and D). To further examine the overall survival rate of PART1, we performed Kaplan–Meier curve analysis and found that higher PART1 expression possessed worse overall survival (Figure 1E). Collectively, we found that lncRNA PART1 was highly expressed in breast cancer cells.
Figure 1.
Long non-coding RNA PART1 expression was elevated in breast cancer cells. (A) The expression of PART1 in breast cancer cell lines and normal breast cell line was examined by qRT-PCR. Breast cancer cell lines: MCF-7, SKBR3, BT-20, MDA-MB-231, ZR-75-1. Normal breast cell line: MCF-10A. (B) The expression of PART1 in breast cancer cell samples and normal tissues was detected using qRT-PCR. n=31. (C) The expression of PART1 in metastasis (n=18) and non-metastasis (n=13) breast cancer samples was detected using qRT-PCR. (D) The expression of PART1 in early stages (n=17) and advanced stages (n=14) breast cancer samples was detected using qRT-PCR. (E) Overall survival rate of PART was analyzed by Kaplan–Meier analysis coupled with long rank test in breast cancer. Higher PART1 expression level group (n=16). Lower PART1 expression level group (n=15). Fold change was normalized to 18S. *P<0.05. All experiments were repeated three times.
Knockdown of PART1 Inhibited the Progression of Breast Cancer Cell
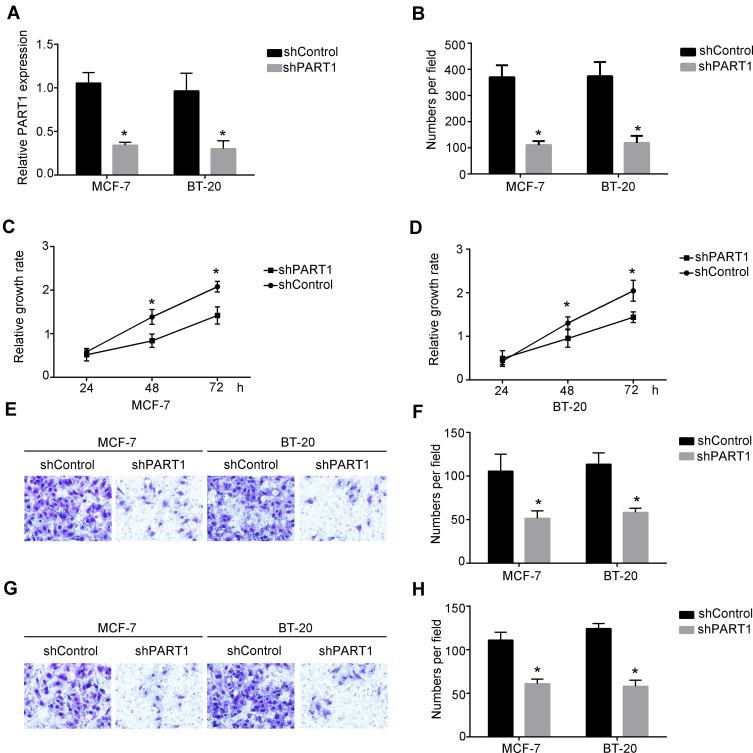
To explore the role of PART1 in breast cancer progression, we constructed the shRNAs specifically targeted lncRNA PART1 (Figure 2A). We found that knockdown of lncRNA PART1 by shRNA in MCF-7 cells significantly decreased the proliferative abilities of MCF-7 cells and BT-20 cells examined by colony formation assay (Figure 2B). Moreover, we observed the same results that knockdown of lncRNA PART1 reduced proliferation of MCF-7 and BT-20 cells examined by CCK-8 assay (Figure 2C and D). Next, we examined that if PART1 regulated the invasion and migration abilities of breast cancer cells. Through transwell assay, we found that knockdown of PART1 decreased the invasion abilities of MCF-7 cells and BT-20 cells (Figure 2E and F). Besides, we also found that downregulation of PART1 inhibited the migration of MCF-7 and BT-20 cells (Figure 2G and H). Taken together, we found that knockdown of PART1 decreased the progression of breast cancer cells.
Figure 2.
Knockdown of PART1 significantly decreased invasion and migration of MCF-7 cells and BT-20 cells. (A) The expression of PART1 after knockdown of PART1 in MCF-7 and BT-20 was detected by qRT-PCR. Fold change was normalized to 18S. (B) Colony formation assay was performed to detect the proliferation of MCF-7 cells and BT-20 cells. (C and D) Cell counting kit-8 assays (CCK-8 assays) were performed to examine the growth curves of MCF-7 cells and BT-20 cells after transfecting with shPART1 plasmid or the negative control. (E and F) Transwell invasion assays were performed to determine the invasion abilities of MCF-7 cells and BT-20 cells after transfection with the negative control or shPART1 plasmid. (G and H) Transwell migration assays were performed to examine the migration of MCF-7 cells and BT-20 cells. *P<0.05. All experiments were repeated three times.
PART1 Targeted miR-4516 Directly
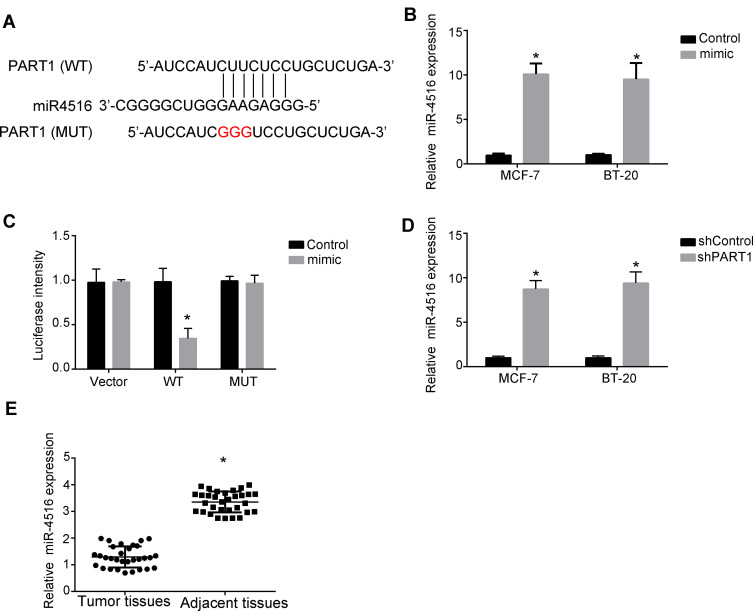
To find the mechanism that PART1 regulated the proliferation, invasion and migration of breast cancer cells, we performed online bioinformatics analysis. We found that PART1 can form complementary base pairing with miR-4516 directly (Figure 3A). miR-4516 was found to regulate many cancers’ progression. However, the potential role of miR-4516 in the regulation of breast cancer remains unknown. To explore the role of miR-4516 in the regulation of PART1-mediated breast cancer progression, we performed miR-4516 overexpression assay (Figure 3B). Then, we performed luciferase reporter assay to verify that PART1 can bind to miR-4516 directly. We found that the luciferase activity was significantly decreased when co-transfecting WT-PART1 and miR-4516 mimics. However, co-transfecting Mutant-PART1 and miR-4516 mimics did not change the luciferase activity (Figure 3C). Besides, we also found that knockdown of PART1 in MCF-7 cells and BT-20 cells increased the expression of miR-4516 examined by qRT-PCR (Figure 3D). As a result, we found that PART1 can direct target miR-4516 and inhibit the expression of miR-4516. Moreover, we also examined the expression of miR-4516 in breast cancer samples. Consistently, we found that the expression of miR-4516 was significantly lower in breast cancer samples than adjacent healthy samples (Figure 3E).
Figure 3.
PART1 binds to miR-4516. (A) The predicted target site between PART1 and miR-4516. (B) Relative expression of miR-4516 was detected using qRT-PCR after overexpression of miR-4516 or not. Fold change was normalized to 18S. (C) The interaction between PART1 and miR-4516 was measured by luciferase reporter assay in MCF-7 cells. MCF-7 cells were co-transfected with the WT reporter plasmid (or the corresponding mutant reporter) and the miR-4516. (D) Relative expression of miR-4516 was detected using qRT-PCR after knockdown of PART1. Fold change was normalized to 18S. (E) Relative expression of miR-4516 was detected using qRT-PCR in breast cancer tissues compared with adjacent healthy tissues. Fold change was normalized to 18S. *P<0.05. All experiments were repeated three times.
PART1 Regulated Breast Cancer Progression Through miR-4516
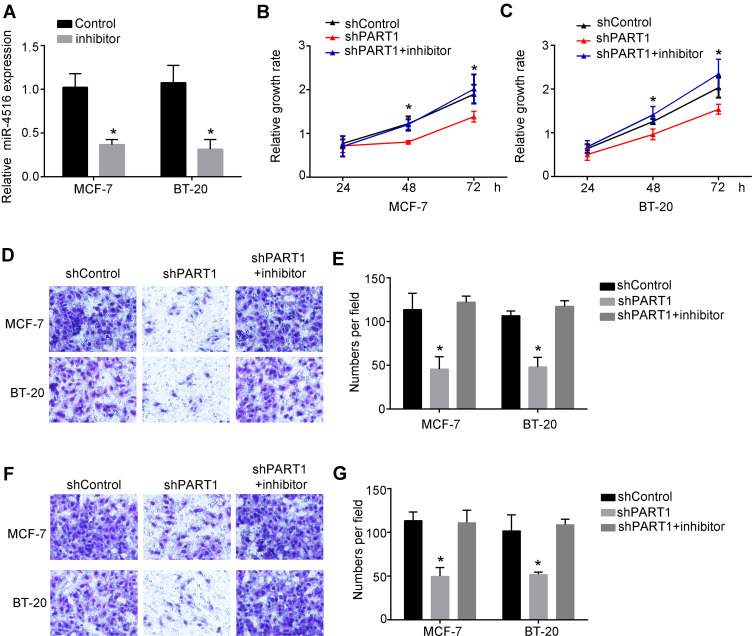
As we showed above, we found that PART1 targeted and inhibited miR-4516. So, we wanted to explore the role of miR-4516 in the regulation of PART1-mediated breast cancer progression. Firstly, we constructed the inhibitor of miR-4516 and verified that inhibition of miR-4516 can decrease the expression of miR-4516 significantly (Figure 4A). To illustrate the biological role of PART1 and miR-4516 in the regulation of breast cancer, we performed functional assay. Through CCK-8 assay, we found that inhibition of miR-4516 can rescue the decreased proliferation abilities of MCF-7 and BT-20 cells caused by knockdown of PART1 (Figure 4B and C). Moreover, we also found that downregulation of PART1 decreased breast cancer cells’ invasion while inhibition of miR-4516 significantly elevated the invasion abilities of breast cancer cells decreased by knockdown of PART1 (Figure 4D and E). Consistently, we found that inhibition of miR-4516 could rescue the decreased migration abilities of MCF-7 and BT-20 cells caused by knockdown of PART1 (Figure 4F and G). Collectively, we showed that PART1 promoted breast cancer cell proliferation, invasion and migration through inhibiting the expression of miR-4516.
Figure 4.
PART1 promoted breast cancer cell progression through inhibiting miR-4516. (A) Relative expression of miR-4516 was detected by qRT-PCR after inhibition of miR-4516. Fold change was normalized to 18S. (B) Relative growth rate of MCF-7 cells and BT-20 cells were detected by Cell Counting Kit-8 (CCK-8) assay. Cells were transfected with shPART1 or shPART1 together with miR-4516 inhibitor or negative control shRNA. (C) Invasion abilities of MCF-7 cells and BT-20 cells were examined by transwell assay. MCF-7 cells and BT-20 cells were transfected with negative control shRNA, shPART1 or shPART1 together with miR-4516 inhibitor. (D and E) Migration abilities of MCF-7 cells and BT-20 cells were examined by transwell assay. MCF-7 cells and BT-20 cells were transfected with negative control shRNA, shPART1 or shPART1 together with miR-4516 inhibitor. (F and G) Invasion abilities of MCF-7 cells and BT-20 cells were examined by transwell assay. MCF-7 cells and BT-20 cells were transfected with negative control shRNA, shPART1 or shPART1 together with miR-4516 inhibitor. *P<0.05. All experiments were repeated three times.
Discussion
In our study, we found that the expression levels of lncRNA PART1 and miR-4516 were higher in breast cancer cell lines compared to normal breast cell lines. Knockdown of PART1 significantly inhibited the proliferation, invasion and migration abilities of breast cancer cells. Moreover, we found that PART1 could bind to miR-4516 and inhibit the expression of miR-4516. Functionally, we found that inhibition of miR-4516 can rescue the decreased progression of breast cancer caused by knockdown of PART1.
lncRNAs were found to play important roles in several biological processes including, development, immunology, and tumorigenesis.22–24 There are numerous evidence to prove that lncRNAs exert vital roles in promoting or inhibiting cancer progression.3–5 And also, a previous study found that lncRNAs can be used as biomarkers for cancers.25 However, there are still many problems needed to be resolved. lncRNA PART1 was found to regulate tumor progression in several cancers, including non-small cell lung cancer, esophageal squamous cell carcinoma, prostate cancer and so on.22–24 However, the role of PART1 in the regulation of breast cancer remains unknown. In our research, we found that PART1 was highly expressed in breast cancer cells and promoted breast cancer progression.
Previous studies have demonstrated that lncRNAs often serve as competitive endogenous RNAs (ceRNAs) to sponge miRNAs and regulate cancer progression.26,27 However, the potential molecular mechanisms need to be explored.26,27 In our study, we made a bioinformatics prediction and found that miR-4516 was a potential target of lncRNA PART1. miR-4516 participates in the regulation of many cancers. For instance, Cui T and colleagues found that miR-4516 predicts poor prognosis of human glioblastoma.17 Li et al found that miR-4516 regulates hepatocellular carcinoma progression through interacting with LSINCT5.18 In our study, we found that miR-4516 can be inhibited by PART1 and inhibition of miR-4516 can promote PART1-mediated breast cancer progression.
lncRNAs and miRNAs exert their role in promoting or inhibiting the progression of cancer cells through target genes. So, it is vital to explore the potential target genes of lncRNA PART1 and miR-4516. According to the previous studies, we found that Chowdhari et al showed miR-4516 can directly target STAT3 protein.19 Moreover, Cui et al demonstrated that miR-4516 binds to PTPN14 and regulates Hippo pathway.17
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Wang O, Yang F, Liu Y, et al. C-MYC-induced upregulation of lncRNA SNHG12 regulates cell proliferation, apoptosis and migration in triple-negative breast cancer. Am J Transl Res. 2017;9(2):533–545. [PMC free article] [PubMed] [Google Scholar]
- 2.Niu J, Li XM, Wang X, et al. DKK1 inhibits breast cancer cell migration and invasion through suppression of β-catenin/MMP7 signaling pathway. Cancer Cell Int. 2019;19:168. doi: 10.1186/s12935-019-0883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu XL, Wang J, He W, Zhao P, Wu WQ. Down-regulation of lncRNA Linc00152 suppressed cell viability, invasion, migration, and epithelial to mesenchymal transition, and reversed chemo-resistance in breast cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(10):3074–3084. doi: 10.26355/eurrev_201805_15067 [DOI] [PubMed] [Google Scholar]
- 4.Wang XX, Guo GC, Qian XK, et al. miR-506 attenuates methylation of lncRNA MEG3 to inhibit migration and invasion of breast cancer cell lines via targeting SP1 and SP3. Cancer Cell Int. 2018;18:171. doi: 10.1186/s12935-018-0642-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao W, Geng D, Li S, Chen Z, Sun M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/HMGA2 axis in breast cancer. Cancer Med. 2018;7(3):842–855. doi: 10.1002/cam4.1353 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Nazir SU, Kumar R, Singh A, et al. Breast cancer invasion and progression by MMP-9 through Ets-1 transcription factor. Gene. 2019;711:143952. doi: 10.1016/j.gene.2019.143952 [DOI] [PubMed] [Google Scholar]
- 7.Cai C, Huo Q, Wang X, Chen B, Yang Q. SNHG16 contributes to breast cancer cell migration by competitively binding miR-98 with E2F5. Biochem Biophys Res Commun. 2017;485(2):272–278. doi: 10.1016/j.bbrc.2017.02.094 [DOI] [PubMed] [Google Scholar]
- 8.Ye B, Liu B, Yang L, et al. LncKdm2b controls self-renewal of embryonic stem cells via activating expression of transcription factor Zbtb3. EMBO J. 2018;37(8). doi: 10.15252/embj.201797174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B, Ye B, Yang L, et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18(5):499–508. doi: 10.1038/ni.3712 [DOI] [PubMed] [Google Scholar]
- 10.Rion N, Ruegg MA. LncRNA-encoded peptides: more than translational noise? Cell Res. 2017;27(5):604–605. doi: 10.1038/cr.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X, Xu X, Sun C, Yu Z. Long intergenic noncoding RNA SNHG16 interacts with miR-195 to promote proliferation, invasion and tumorigenesis in hepatocellular carcinoma. Exp Cell Res. 2019;383:111501. doi: 10.1016/j.yexcr.2019.111501 [DOI] [PubMed] [Google Scholar]
- 12.Li H, Ma SQ, Huang J, Chen XP, Zhou HH. Roles of long noncoding RNAs in colorectal cancer metastasis. Oncotarget. 2017;8:39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Zhou J, Wang Z, Wang P, Li S. Upregulation of SOX2 activated LncRNA PVT1 expression promotes breast cancer cell growth and invasion. Biochem Biophys Res Commun. 2017;493(1):429–436. doi: 10.1016/j.bbrc.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 14.Zhao C, Ling X, Li X, Hou X, Zhao D. MicroRNA-138-5p inhibits cell migration, invasion and EMT in breast cancer by directly targeting RHBDD1. Breast Cancer. 2019;26(6):817–825. doi: 10.1007/s12282-019-00989-w [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Liu H, Li M. Downregulation of miR-505 promotes cell proliferation, migration and invasion, and predicts poor prognosis in breast cancer. Oncol Lett. 2019;18(1):247–254. doi: 10.3892/ol.2019.10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu M, Gou L, Xia J, et al. LncRNA ITGB2-AS1 could promote the migration and invasion of breast cancer cells through up-regulating ITGB2. Int J Mol Sci. 2018;19(7):1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui T, Bell EH, McElroy J, et al. miR-4516 predicts poor prognosis and functions as a novel oncogene via targeting PTPN14 in human glioblastoma. Oncogene. 2019;38(16):2923–2936. doi: 10.1038/s41388-018-0601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li O, Li Z, Tang Q, et al. Long stress induced non-coding transcripts 5 (LSINCT5) promotes hepatocellular carcinoma progression through interaction with high-mobility group AT-hook 2 and MiR-4516. Med Sci Mon Int Med J Exp Clin Res. 2018;24:8510–8523. doi: 10.12659/MSM.911179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. J Cell Physiol. 2014;229(11):1630–1638. doi: 10.1002/jcp.24608 [DOI] [PubMed] [Google Scholar]
- 20.Kong Q, Qiu M. Long noncoding RNA SNHG15 promotes human breast cancer proliferation, migration and invasion by sponging miR-211-3p. Biochem Biophys Res Commun. 2018;495(2):1594–1600. doi: 10.1016/j.bbrc.2017.12.013 [DOI] [PubMed] [Google Scholar]
- 21.Li R, Zhu H, Yang D, Xia J, Zheng Z. Long noncoding RNA lncBRM promotes proliferation and invasion of colorectal cancer by sponging miR-204-3p and upregulating TPT1. Biochem Biophys Res Commun. 2019;508(4):1259–1263. doi: 10.1016/j.bbrc.2018.12.053 [DOI] [PubMed] [Google Scholar]
- 22.Li M, Zhang W, Zhang S, Wang C, Lin Y. PART1 expression is associated with poor prognosis and tumor recurrence in stage I-III non-small cell lung cancer. J Cancer. 2017;8(10):1795–1800. doi: 10.7150/jca.18848 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Sun M, Geng D, Li S, Chen Z, Zhao W. LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol Chem. 2018;399(4):387–395. doi: 10.1515/hsz-2017-0255 [DOI] [PubMed] [Google Scholar]
- 24.Kang M, Ren M, Li Y, Fu Y, Deng M, Li C. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res. 2018;37(1):171. doi: 10.1186/s13046-018-0845-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Huang C, Liu S, Wang H, Zhang Z, Yang Q, Gao F. LncRNA PVT1 overexpression is a poor prognostic biomarker and regulates migration and invasion in small cell lung cancer. Am J Transl Res. 2016;8(11):5025–5034. [PMC free article] [PubMed] [Google Scholar]
- 26.Abdollahzadeh R, Daraei A, Mansoori Y, Sepahvand M, Amoli MM, Tavakkoly-Bazzaz J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: a new look at hallmarks of breast cancer. J Cell Physiol. 2019;234(7):10080–10100. doi: 10.1002/jcp.27941 [DOI] [PubMed] [Google Scholar]
- 27.Jiang R, Zhao C, Gao B, Xu J, Song W, Shi P. Mixomics analysis of breast cancer: long non-coding RNA linc01561 acts as ceRNA involved in the progression of breast cancer. Int J Biochem Cell Biol. 2018;102:1–9. doi: 10.1016/j.biocel.2018.06.003 [DOI] [PubMed] [Google Scholar]