Abstract
Background
Retinopathy of prematurity (ROP) is a widely recognized cause of blindness after preterm birth. The incidence of ROP is rising especially in low- and middle-income countries (LMIC) because of improved neonatal care and increased survival of very premature neonates. To date, there is no data on incidence of ROP in Botswana.
Objective
The purpose of this study was to provide initial data and determine ROP-associated risk factors from a single neonatal care center on the incidence of ROP in Gaborone, Botswana.
Methods
A prospective observational study was conducted at Princess Marina Hospital (PMH) in Gaborone, Botswana. Premature neonates with birth weights (BW) of <1,801 g or gestational age (GA) of <34 weeks were enrolled in this study. Diagnostic examinations were conducted using an indirect ophthalmoscope with 28D lens. ROP findings were classified according to the most advanced stage of ROP reached using the International Classification of ROP (2005). Data were entered into STATA version 15 statistical software for analysis.
Results
There were 264 premature infants enrolled in the study. ROP screening was performed on 200 (75.8%). Of all 264 enrolled patients 133 (50.4%) were female. The mean GA was 30.3±2.6 (range 24–37) weeks and the mean BW was 1302.2±285.9 g (range 725–2035). Out of 200 who were screened, we identified 22 with ROP with a ROP incidence of 11%. The incidence of type 1 ROP (sight-threatening) was found to be 3.5%. This study identified a significant difference in possible ROP risk factors between those infants who develop ROP and those who do not, eg, BW (p<0.001), GA (p=0.024) and blood transfusion (p=0.001).
Conclusion
This study demonstrates that ROP is a treatable cause of blindness in Botswana. Lack of a proper screening protocol, delay in diagnosis and management are plausible reasons for poor outcome in those who were diagnosed with type 1 ROP.
Keywords: retinopathy of prematurity, gestational age, birth weight, type 1 ROP, type 2 ROP
Introduction
Retinopathy of prematurity (ROP), a vasoproliferative disorder affecting the retina of premature babies, is a potentially avoidable cause of blindness if its severe forms are detected and treated in a timely manner. It is a widely recognized cause of visual impairment after preterm birth. The proportion of blindness as a result of ROP varies greatly among countries depending on their level of development, being influenced by the availability of neonatal care, neonatal outcomes, and whether effective screening and treatment programs are in place.1 It is essential that ROP surveillance programs are developed specifically for each region or country.
Larger, more mature infants develop severe ROP in countries with low/moderate levels of economic development (LMIC) when compared with high income countries (HIC) where the population of infants currently at risk for severe ROP requiring treatment is extremely premature, with birth weights almost always <1,000 g.2 The incidence of ROP blindness in certain Latin American and East European countries has recently been as high as 38.6 and 25.9%, respectively.3 This increased incidence of ROP blindness in countries with developing neonatal intensive care systems and improving survival of premature infants has been designated the “third epidemic” of ROP blindness.3 Higher birth rate and increased rate of premature birth, compromised neonatal care as a result of lack of resources, absence of clear screening guidelines and lack of skilled manpower to screen and identify those at risk were reasons for the higher prevalence rate in middle income countries.4 For example, there seems to be a more liberal use of oxygen supplementation with oxygen saturations up to 100% at the neonatal unit at Princess Marina Hospital, as compared to European units. Recent oxygen saturation targeting trials show increased mortality with lower target ranges. Target ranges above 88% saturation are currently suggested for premature infants.5 According to a meta-analyses on oxygenation saturation targeting trials, a 91–95% range can be recommended for all extremely preterm infants from birth but should be accompanied by stringent surveillance for the prevention and early treatment of ROP.6
Studies done in a South African tertiary hospital have shown the incidence of ROP to be 16.3% with 1.6% of the patients diagnosed with severe ROP.7 Currently in West Africa, the major anatomical cause of severe visual impairment/blindness is reported to be corneal scar and phthisis bulbi (35.9%). Retinal disease accounted for 20.4%, cataract 15.5%, glaucoma 13.0% and blindness from ROP is currently rarely reported.8 There is also no data on the prevalence of ROP in Botswana. The purpose of this study was to provide initial data and determine ROP-associated risk factors from a single neonatal care center on the incidence of ROP in Gaborone, Botswana.
Research Method and Design
This study was performed at the Princess Marina Hospital (PMH) in Gaborone, Botswana. This tertiary care neonatal unit (NNU) has 39 beds including a 6-bed neonatal intensive care unit (NICU). The unit admits neonates referred in house from the labor ward and operation theatre in PMH as well as externally from home, all local clinics and surrounding district hospitals in the PMH catchment area. The neonatal unit admits an average of 98 neonates per month. Of these 49 are neonates whose gestational age is less than 37 weeks. Premature neonates with birth weights of <1,801 g or gestational age (GA) of <34 weeks were eligible for enrollment in this study. This classification of birth weight and gestational age was made after modifying the British ROP screening criteria.9 In addition, based on the transfers of critically ill children into the NICU, survival beyond 48 hours was required for eligibility. Informed consent was sought from parents of neonates meeting these criteria. Neonates with congenital ophthalmic malformations and those parents who did not consent for the study were excluded. This project was approved by institutional review committees of University of Botswana, Ministry of Health and Wellness of Botswana, Princess Marina Hospital and University of Pennsylvania.
Ophthalmologic Examinations
ROP diagnostic examinations were conducted by an ophthalmologist (JS) trained in detection of ROP through 15 years. After pupillary dilatation using cyclopentolate 1% (MINIMIS Bausch & Lomb, iNova Pharmaceuticals) and tropicamide 10 mg/mL eye drops (MYDRIACYL, Alcon laboratories (SA)) the fundus was examined using an indirect ophthalmoscope (YZ25B Binocular indirect ophthalmoscope, People's Republic of China) with 28D lens and scleral indentation. Local anesthetic agent and Flynn lid speculum were used as needed. ROP findings were classified according to the most advanced stage of ROP reached using the International Classification of ROP (2005).10 ROP was defined and graded according to the appearance of the most severe form of ROP. In brief; type 1 ROP is defined as the most severely affected with zone I, any stage ROP with plus disease, zone I, stage 3.4 and 5 ROP with or without plus disease, and zone II, stage 2 or 3, 4, 5 with plus disease. Type 2 ROP was accordingly defined as zone 1 stage 1 or 2 without plus disease and zone 2 stage 3 without plus disease.
Timing of initial examinations were determined based on the GA of the infant. For infants with <27 weeks, ROP examinations had been undertaken at 30–31 weeks postmenstrual age (PMA). For infants born between 27 and 34 weeks, ROP diagnostic examination had been undertaken between 4 and 5 weeks postnatal age. For infants with >34 weeks GA, but BW <1,801 g, the first ROP examination had been undertaken between 4 and 5 weeks postnatal age. For neonates who remained in the neonatal unit of PMH because of their illness, the first ROP screening was conducted in the neonatal unit. Those who were discharged before the ROP screening date were given an appointment date and indirect ophthalmoscopy was done when they arrived at the outpatient department at the PMH eye clinic. The appointment date was sometimes accidentally delayed beyond the preferred follow-up date. Subsequent follow-ups were determined by the presence or absence of ROP and determined in accordance with the screening protocol below. Weekly examinations were indicated if vascularization extended only into zone I or II, plus or pre plus disease was noted, or if stage 3 ROP was noted. Two-weekly examinations were conducted until the criteria for termination of surveillance was reached. These criteria were:
For infants without ROP, vascularization had extended well into zone III, usually after 36 weeks PMA.
For infants found to have ROP, examination was discontinued when treatment is indicated or any of the following characteristics of regression were observed on at least two successive examinations:
a) no increase in severity
b) regressing ROP noted such as a change in color of ridge, retinal vessels proceeding beyond the ridge.
Premature neonates who developed a severe form of ROP were referred to an ophthalmology treatment center in South Africa as there was no facility (public or private) which can provide treatment in Botswana.
Risk factors were recorded for each infant including maternal HIV status, mode of delivery, multiple birth, GA, BW, clinical conditions such as respiratory distress, sepsis, intraventricular hemorrhage, anemia, blood transfusion and exposure to phototherapy.
Data Analysis
Data were entered into STATA version 15 statistical software for analysis. Descriptive statistics included the mean and standard deviation for numerical variables, and frequency and percentage of categorical variables. The incidence rate of ROP was described in simple proportion. Group comparisons were done by Fisher’s exact chi-squared test. A probability (P) of less than 0.05 was considered statistically significant.
Results
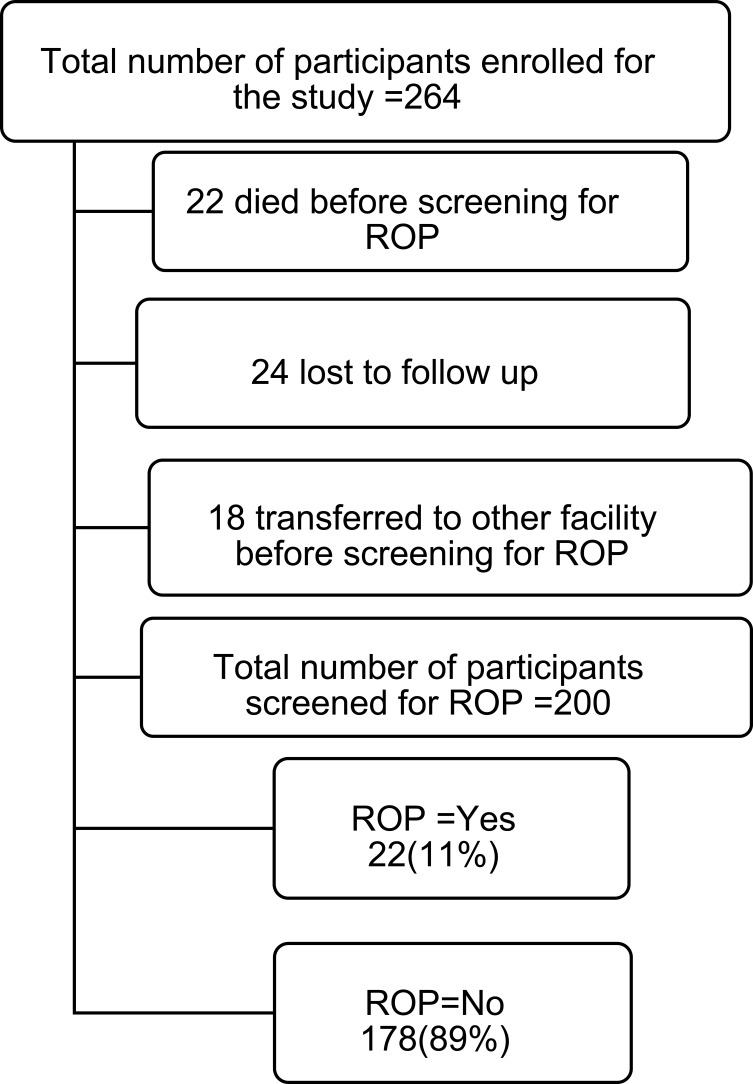
There were 264 infants enrolled during the study period from June 1, 2018 to May 30, 2019. ROP screening was performed on 200 (75.8%) of these infants. Twenty-two (8.4%) had died before the first ophthalmologic screening and 24 (9.1%) were lost to follow up. An additional, 18 infants (6.9%) were transferred to other health facilities before the first screening (Figure 1).
Figure 1.
Study participants enrolled, screened for ROP,died and lost from follow up.
Of all 264 enrolled patients, 133 (50.4%) were female. The mean (SD) GA was 30.3±2.6 (range 24–37) weeks and the mean (SD) BW was 1302.2±285.9 g (range 725–2035). The majority (92%) of infants were born in a health facility. Multiple gestation was observed in 27 (10.2%) of the cases. Sixty-nine (26.1%) of enrolled infants were born to HIV-positive mothers (Table 1).
Table 1.
Demographic Characteristic of Premature Neonates Enrolled for Screening of Retinopathy of Prematurity
| Variable | Frequency (Percent) |
|---|---|
| Birth Weight (grams) | |
| 500–750 | 3 (1.1) |
| 751–900 | 16 (6.1) |
| 901–1000 | 21 (8.0) |
| 1001–1100 | 31 (11.7) |
| 1101–1250 | 44 (16.7) |
| 1251–1500 | 68 (25.8) |
| 1501–1700 | 48 (18.2) |
| >1700 | 19 (7.2) |
| Missing | 14 (5.3) |
| Mean (SD) | 1302.21 (285.9) |
| (Min, Max) | (725,2035) |
| Gestational Age (weeks) | |
| ≤26 | 19 (7.2) |
| 27 | 27 (10.2) |
| 28 | 26 (9.9) |
| 29 | 36 (13.6) |
| 30 | 28 (10.6) |
| 31 | 31 (11.7) |
| 32 | 33 (12.5) |
| 33 | 22 (8.3) |
| ≥34 | 33 (12.5) |
| Missing | 9 (3.4) |
| Mean (SD) | 30.28 (2.6) |
| (Min, Max) | (24,37) |
| Gender | |
| Male | 124 (47.0) |
| Female | 133 (50.4) |
| Missing | 7 (2.7) |
| Birth Location | |
| Inborn (Institutional) | 243 (92.1) |
| Out born (home) | 8 (3.0) |
| Missing | 11 (4.2) |
| Multiple Gestation | |
| Yes | 27 (10.2) |
| No | 228 (86.4) |
| Missing | 9 (3.4) |
| Maternal HIV Status | |
| HIV positive | 69 (26.1) |
| HIV negative | 178 (67.4) |
| Unknown status | 9 (3.4) |
| Missing | 8 (3.0) |
The most common diagnoses on admission were sepsis (58.7%), respiratory distress syndrome (40.2%), hypothermia (40.2%) and hypoglycemia (13.6%). Only 42 (15.9) premature babies were admitted into the NICU. Thirty-six (13.6%) of the enrolled premature infants received a blood transfusion (Table 2). Out of 200 who were screened for ROP during the 12 months of the study, we identified 22 with ROP with an ROP incidence of 11%. The incidence of type 1 ROP (sight threatening) was found to be 3.5% (Table 3).
Table 2.
Clinical Characteristics of Premature Neonates Enrolled for Screening of Retinopathy of Prematurity
| Variables | Frequency (Percent) |
|---|---|
| Admission Diagnosis | |
| Sepsis | 155 (58.7) |
| Respiratory distress syndrome (RDS) | 106 (40.2) |
| Hypothermia | 106 (40.2) |
| Hypoglycemia | 36 (13.6) |
| Neonatal jaundice | 27 (10.2) |
| Congenital pneumonia | 20 (7.6) |
| Admission in ICU | |
| No | 202 (84.1) |
| Yes | 42 (15.9) |
| Frequency of Blood Transfusion | |
| Once | 14 (5.3) |
| Multiple | 22 (8.3) |
| None | 217 (82.2) |
| Missing | 11 (4.2) |
| Number of Days on Photo Therapy | |
| <1 | 7 (2.8) |
| 1–3 | 41 (16.4) |
| ≥4 | 22 (8.8) |
| None | 180 (72.0) |
| Missing | 14 (5.3) |
Table 3.
Screening Outcome of Premature Neonates Enrolled for Study on Retinopathy of Prematurity
| Variables | Frequency (Percent) |
|---|---|
| ROP Statusa | |
| Type 1 ROPb | 7 (3.5) |
| Type 2 ROPc | 1 (0.5) |
| Not type 1 or 2 ROP | 14 (7.0) |
| No ROP | 178 (89.0) |
Notes: aROP by worse outcome for an infant, bType 1 ROP is: Zone I, any stage ROP with plus disease. Zone I, stage 3 and above ROP (4, 5) with or without plus disease. Zone II, stage 2 or 3, 4, 5 with plus disease. cTypE 2 ROP. Zone 1 stage 1 or 2 without plus disease. Zone 2 stage 3 without plus disease.
Table 4 depicts the stage, zone and pre plus, plus and aggressive posterior ROP in those diagnosed with ROP.
Table 4.
Stage, Zone and Presence of Pre Plus, Plus and Aggressive Posterior ROP in Premature Neonates Diagnosed with ROP
| Variable | Right Eye n=22 n (%) |
Left Eye n=22 n (%) |
|---|---|---|
| Stage | ||
| 1 | 8 (36.4) | 8 (36.4) |
| 2 | 7 (31.8) | 7 (31.8) |
| 3 | 4 (18.2) | 4 (18.2) |
| 4A | 1 (4.5) | 2 (9.1) |
| 4B | 1 (4.5) | 0 (0.0) |
| 5 | 1 (4.5) | 1 (4.5) |
| Zone | ||
| 1 | 4 (18.2) | 4 (18.2) |
| 2 | 16 (72.7) | 16 (72.7) |
| 3 | 2 (9.1) | 2 (9.1) |
| Pre plus disease | 0 (0.0) | 0 (0.0) |
| Plus disease | 4 (18.2) | 4 (18.2) |
| Aggressive posterior (AP-ROP) | 0 (0.0) | 0 (0.0) |
The majority of examined infants who developed ROP were < 28 weeks of GA and <1,000 g BW. However, ROP was also observed in those whose GA was >32 weeks and BW of >1,250 g.Those who had developed type 1 ROP in one or both eyes were born at GA of 26–31 weeks and had a BW of 800–1,150 g. This study demonstrates a significant difference in possible ROP risk factors between those infants who develop ROP and those who do not, eg, BW (p<0.001), GA (p=0.024) and blood transfusion (p=0.001) (Table 5).
Table 5.
Factors That Could Be Associated with Development of ROP in Premature Neonates Enrolled for Screening of Retinopathy of Prematurity
| Variable | ROP Frequency (Percent) | No ROP Frequency (Percent) | p-value |
|---|---|---|---|
| Gestational Age (weeks) | 0.024 | ||
| <26 | 3 (16.7) | 15 (83.3) | |
| 27 | 3 (15.8) | 16 (84.2) | |
| 28 | 7 (36.8) | 12 (63.2) | |
| 29 | 2 (6.9) | 27 (93.1) | |
| 30 | 2 (4.5) | 21 (95.5) | |
| 31 | 2 (8.3) | 22 (91.7) | |
| 32 | 1 (4.3) | 22 (95.7) | |
| 33 | 1 (6.3) | 15 (93.8) | |
| ≥34 | 1 (5.0) | 19 (95.0) | |
| Missing | 0 (0.0) | 9 (5.3) | |
| Birth Weight (g) | <0.001 | ||
| 500–750 | 2 (66.7) | 1 (33.3) | |
| 751–900 | 4 (36.4) | 7 (63.6) | |
| 901–1000 | 6 (33.3) | 12 (66.7) | |
| 1001–1100 | 3 (11.5) | 23 (88.5) | |
| 1101–1250 | 2 (5.7) | 33 (94.3) | |
| 1251–1500 | 3 (5.9) | 48 (94.1) | |
| 1501–1700 | 1 (3.3) | 29 (96.7) | |
| >1700 | 0 (0.0) | 12 (100.0) | |
| Missing | 1 (4.5) | 12 (6.7) | |
| Gender | 0.488 | ||
| Male | 8 (8.8) | 83 (91.2) | |
| Female | 14 (12.9) | 88 (87.1) | |
| Missing | 0 | 6 (3.4) | |
| Admission in ICU | 0.139 | ||
| Yes | 6 (14.3) | 36 (85.8) | |
| No | 16 (7.3) | 142 (92.7) | |
| Place of Delivery | 0.517 | ||
| Institutional (inborn) | 22 (12.2) | 158 (87.8) | |
| Home (out born) | 0 (0.0) | 8 (100.0) | |
| Other | 0 (0.0) | 2 (100.0) | |
| Missing | 0 | 10 (5.6) | |
| Admission Diagnosis | |||
| Sepsis | 6 (7.9) | 70 (92.1) | 0.354 |
| RDS | 10 (11.6) | 76 (88.4) | 0.817 |
| Hypothermia | 10(12.8) | 68 (88.2) | 0.482 |
| Hypoglycemia | 4 (15.4) | 22 (84.6) | 0.491 |
| Neonatal jaundice | 3 (13.0) | 20 (87.0) | 0.718 |
| Congenital pneumonia | 0 (0.0) | 18 (100.0) | 0.227 |
| Frequency of Blood Transfusion | 0.001 | ||
| Once | 5 (35.7) | 9 (64.3) | |
| Multiple | 4 (18.2) | 16 (81.8) | |
| None | 12 (7.8) | 142 (92.2) | |
| Missing | 1 (4.5) | 11 (6.2) | |
| Number of Days on Photo Therapy | 0.308 | ||
| <1 | 1 (16.7) | 5 (83.3) | |
| 1–3 | 7 (18.9) | 30 (81.1) | |
| ≥4 | 3 (14.3) | 18 (85.7) | |
| None | 10 (8.3) | 111 (91.7) | |
| Missing | 1 (4.5) | 14 (7.9) | |
| Maternal HIV Status | 0.843 | ||
| HIV positive | 6 (11.1) | 48 (88.9) | |
| HIV negative | 15 (11.3) | 118 (88.7) | |
| Unknown status | 1 (16.7) | 5 (83.3) |
The seven infants diagnosed with type 1 ROP were referred to the Republic of South Africa (RSA) for further management as there was no facility to treat these patients in Botswana at that time. All received treatment at the South African treatment center at about 15 days delay, after the consultation and confirmation of ROP in Botswana. Five of the patients had already developed tractional retinal detachment by the time they arrived in RSA for treatment. Only two of the seven patients did undergo pars plana vitrectomy (PPV), while the rest were deemed to have poor prognosis and no intervention was given. One patient who was diagnosed with type 2 ROP had progressed to type 1 (Table 6).
Table 6.
Clinical Characteristics and Outcome of Type 1 ROP in Infants Screened
| Serial Number | Gestational Age in Weeks | Birth Weight in Grams | Place of Delivery | Retinal Findings | Treatment Given | |||
|---|---|---|---|---|---|---|---|---|
| Inborn | Out Born | Left Eye | Right Eye | Left Eye | Right Eye | |||
| 1 | 27 | 800 | Yes | TRDb | TRD | None | None | |
| 2 | 30 | 930 | Yes | Stage 4 | TRD | PPVa | None | |
| 3 | 26 | 770 | Yes | TRD | TRD | None | None | |
| 4 | 28 | 950 | Yes | TRD | TRD | None | None | |
| 5 | 31 | 1150 | Yes | TRD | TRD | None | None | |
| 6 | 27 | 855 | Yes | TRD | TRD | None | None | |
| 7 | 28 | 1065 | Yes | Stage 4 | Stage 4 | PPV | PPV | |
Abbreviations: aPPV, pars plana vitrectomy; bTRD, tractional retinal detachment.
Discussion
Retinopathy of prematurity is one of the most important treatable causes of blindness in children. Studies on ROP in sub-Saharan Africa are scanty and the magnitude of the problem has not been well documented. We embarked on this study of ROP in one of Botswana’s two referral hospitals to estimate the incidence of ROP in the south of the country. This information will inform policy and practice in the management of ROP in Botswana. In our cohort from a single tertiary center in Botswana’s capital, we have documented a ROP incidence of 11% while the incidence of sight- threatening ROP was 3.5%. The incidence of ROP in our study is lower than that reported in South Africa and other LMIC. Keraan et al,11 from Cape Town, South Africa, in a cohort of 159 patients reported an incidence of all forms of ROP, all grades, of 29.6% and severe ROP of 5.9%. Similarly, Mayet and Cockinos7 from South Africa reported a prevalence of 16.3% and of severe ROP 1.6%. Bassiouny et al,12 reported an incidence of 34%, while Maheshwari et al13 reported an incidence of 27% in India. We speculate that the lower incidence in our study may be attributed to the high mortality of premature infants who were not screened for ROP, and the PMH neonatal unit policy not to provide intensive care treatment for premature infants with a birth weight (BW) below 900 g. A master thesis survey in our unit included patients from January 2015 to December 2017 and documented that only 25% of babies with a BW below 900 g survive (unpublished data, MMed thesis, Thlaodi et al). In the present study 19 screened patients had a BW < 900 g (7% of all) vs 245 with a BW > 900 g. Three of the seven patients diagnosed with severe ROP had a BW< 900 g (Table 5). These, according to department policy, were only offered a warm bed, fluids, peroral nutrition and liberale oxygen supplementation, and no further advanced intensive care treatment such as respiratory support, observations and documentation of oxygen supplementation and saturation measurements. In spite of this, only 6/19 developed ROP and 3/19 (16%) severe ROP. An obvious limitation of this study is the lack of detailed clinical observations including measures to identify controlled oxygen supplementation and treatment for babies with a birth weight less than 900 g who constitute 7% of those screened for ROP.
Lower BW and GA are associated with higher incidences of ROP, in an inverse relationship.14 This was confirmed in our study where 13 (59.1%) of the infants diagnosed with ROP had a GA <28 weeks and 12 (54.5%) BW <1,000 g. These findings are similar to those reported in both high income countries (HIC) and LMIC. According to Gilbert et al, mean GAs of infants with severe ROP in highly developed countries were <26 weeks, which is lower than GAs reported in LMIC which ranged from 26.3 weeks in Lithuania to 33.5 weeks in Ecuador.3 The mean birth weights of infants who develop severe ROP in HIC ranged from 737 to 763 g compared with values of 903 to 1527 g in LMIC.2 Blood transfusion was also associated with a higher incidence of ROP. This association has been reported in studies of ROP globally.15,16 Apart from the association between increased risk of preterm birth we were not able to find any association between HIV exposure and ROP in preterm infants.17
In our cohort we identified more severe forms of ROP in 7 (3.5%) of the screened patients. Five had developed retinal detachment by the time they arrived at the ophthalmic treatment center. The main reasons for this catastrophe were 1) delay in screening at the eye clinic; and 2) delay in referral for treatment in South Africa, as there are no treatment centers in Botswana. All of those referred for treatment reached the South African treatment center after 15 days. The delay is usually caused by the logistics of funding and preparation of travel documents. The current recommendation is that treatment should be done within 72 hours of diagnosis of type 1 ROP.18 Two of the patients subsequently underwent posterior pars plana vitrectomy for tractional retinal detachment with poor visual outcome. One of the patients who was diagnosed with type 2 ROP progressed to type 1 ROP. However, because of delay in the referral system the patient did not arrive on time for intervention and subsequently developed a retinal detachment; no intervention was carried out because of poor visual prognosis. ROP regressed in the remaining 14 (7%) patients during the follow-up period.
Our study has limitations because many patients were lost to follow up or transferred out of Princess Marina Hospital before undergoing screening. There was a high mortality rate among premature neonates, especially those with extremely low birth weight and immaturity. These were not offered intensive care and in these babies the incidence of ROP was highest. Unavailability of oxygen blenders and medical equipment to monitor fraction of oxygen (FiO2) concentration given to sick premature babies added to the challenge to identify the effect and magnitude of oxygen use and risk of ROP. Furthermore, lack of a standard referral system, ROP screening protocols and availability of in-country treatment resulted in catastrophic outcomes in those who were diagnosed with severe ROP at the tertiary center in Gaborone but delayed for appropriate treatment.
Conclusion
This study demonstrated that ROP is a treatable cause of blindness in Botswana. Lack of a proper screening protocol, delay in diagnosis and management are plausible reasons for poor outcome in those who were diagnosed with treatable ROP.
In order to reduce ROP related blindness we recommend:
Increase the awareness of policy makers and health professionals of the magnitude of ROP.
Develop ROP screening protocols and advocate for their inclusion in the integrated management of childhood disease (IMCI) and the Botswana national prevention of blindness plan.
Liaise with relevant governmental authorities to consider digital retinal screening technology using telemedicine in Botswana.
Advocate for availability of ROP screening and treatment services in the new teaching hospital.
Acknowledgment
The authors would like to acknowledge the following for substantial contribution to the success of this research project: the Portor ladies for their support to the project; the Center for Global Health at the Children’s Hospital of Philadelphia for availing a grant for the research project; the parents and patients who consented to participate in the study without whom this may not be a reality; Dr Balebanye Thlaodi for allowing us to use mortality data from her master thesis. We also would like to thank Dr Thuso David for translating the patient consent form from English to Setswana and Dr Loeto Mazhani for valuable support during undertaking of this research.
The abstract of this paper was presented at the Ophthalmological Society of Ethiopia 21st annual scientific conference and health exhibition held on September 12–20, 2019 as a conference talk with interim findings. The abstract poster was published on research gate platform. https://www.researchgate.net/publication/338459109_Retinopathy_of_prematurity_incidence_and_risk_factors_in_premature_infants_admitted_in_neonatal_unit_of_a_tertiary_hospital_in_Botswana
Funding Statement
Financial support was received from the Global Health pilot grant from the Children’s Hospital of Philadelphia for hiring research coordinator, pay transport costs of study participants and covering costs for conference presentation, as well as to cover the costs of publication. The Institute had no role in the design of the study, collection, analysis, and interpretation of the data, or in writing of the manuscript.
Consent for Publication
The parents/legal guardians of all minor participants used in the study provided informed, written consent for the publication of identifiable personal/clinical details within this study. They all agreed and gave permission for the authors for the contents of this study to be published or presented to scientific journals or scientific conferences.
Abbreviations
BW, birth weight; GA, gestational age; HIC, high income countries; LMIC, low- and middle-income countries; NICU, neonatal intensive care unit; PMA, post menstrual age; ROP, retinopathy of prematurity; TRD, total retinal detachment.
Ethics Approval and Consent to Participate
The research protocol adhered to the guidelines of the Declaration of Helsinki. The Institutional review board of the University of Botswana (UBR/RES/IRB/1754,29/11/2016), the Ministry of Health and Wellness of Botswana (HPDME:13/18/1 VOL. X(895),23/01/2017), Princess Marina Hospital (PMH 5/79 (330-2-2017), 20/7/2017), and University of Pennsylvania (IRB Protocol #829,671,04/05/2018) granted approval for this research. All infants screened were asked to participate, and informed written consent was provided by their parents.
Disclosure
The authors report no conflicts of interest.
References
- 1.Liu PM, Fang PC, Huang CB, et al. Risk factors of retinopathy of prematurity in premature infants weighing less than 1600 g. Am J Perinatol. 2005;22(2):115–120. doi: 10.1055/s-2005-837276 [DOI] [PubMed] [Google Scholar]
- 2.Gilbert C, Fielder A, Gordillo L, et al. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: implications for screening programs. Pediatrics. 2005;115(5):e518–e525. doi: 10.1542/peds.2004-1180 [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C, Rahi J, Eckstein M, O’Sullivan J, Foster A. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350(9070):12–14. doi: 10.1016/S0140-6736(97)01107-0 [DOI] [PubMed] [Google Scholar]
- 4.Fortes Filho JB, Eckert GU, Procianoy L, Barros CK, Procianoy RS. Incidence and risk factors for retinopathy of prematurity in very low and in extremely low birth weight infants in a unit-based approach in southern Brazil. Eye. 2009;23(1):25–30. doi: 10.1038/sj.eye.6702924 [DOI] [PubMed] [Google Scholar]
- 5.Higgins RD. Oxygen saturation and retinopathy of prematurity. Clin Perinatol. 2019;46(3):593–599. doi: 10.1016/j.clp.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 6.Askie LM. Meta-analysis of oxygenation saturation targeting trials: do infant subgroups matter? Clin Perinatol. 2019;46(3):579–591. doi: 10.1016/j.clp.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 7.Mayet I, Cockinos C. Retinopathy of prematurity in South Africans at a tertiary hospital: a prospective study. Eye. 2006;20(1):29–31. doi: 10.1038/sj.eye.6701779 [DOI] [PubMed] [Google Scholar]
- 8.Gilbert CE, Canovas R, Hagan M, Rao S, Foster A. Causes of childhood blindness: results from West Africa, South India and Chile. Eye. 1993;7(1):184–188. doi: 10.1038/eye.1993.39 [DOI] [PubMed] [Google Scholar]
- 9.Wilkinson AR, Haines L, Head K, Fielder AR. UK retinopathy of prematurity guideline. Early Hum Dev. 2008;84(2):71–74. doi: 10.1016/j.earlhumdev.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 10.Quinn GE. The international classification of retinopathy of prematurity revisited: an international committee for the classification of retinopathy of prematurity. Arch Ophthalmol. 2005;123(7):991–999. [DOI] [PubMed] [Google Scholar]
- 11.Keraan Q, Tinley C, Horn A, Pollock T, Steffen J, Joolay Y. Retinopathy of prematurity in a cohort of neonates at Groote Schuur Hospital, Cape Town, South Africa. South African Med J. 2017;107(1):64–69. doi: 10.7196/SAMJ.2017.v107i1.11226 [DOI] [PubMed] [Google Scholar]
- 12.Maheshwari R, Kumar H, Paul VK, Singh M, Deorari AK, Tiwari HK. Incidence and risk factors of retinopathy of prematurity in a tertiary care newborn unit in New Delhi. Nat Med J India. 1996;9(5):211–214. [PubMed] [Google Scholar]
- 13.Bassiouny MR. Risk factors associated with retinopathy of prematurity: a study from Oman. J Trop Pediatr. 1996;42(6):355–358. doi: 10.1093/tropej/42.6.355 [DOI] [PubMed] [Google Scholar]
- 14.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(SUPPL. 1):35–49. doi: 10.1038/pr.2013.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakeem A, Mohamed G, Othman M. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol. 2012;19(3):289–294. doi: 10.4103/0974-9233.97927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdel Hadi AM, Hamdy IS. Correlation between risk factors during the neonatal period and appearance of retinopathy of prematurity in preterm infants in neonatal intensive care units in Alexandria, Egypt. Clin Ophthalmol. 2013;7:831–837. doi: 10.2147/OPTH.S40136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stringer EM, Kendall MA, Lockman S, et al. Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS One. 2018;13(7):e0199555. doi: 10.1371/journal.pone.0199555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Good WV; Group ET for R of PC. Final results of the early treatment for retinopathy of prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233–48; discussion 248–50. [PMC free article] [PubMed] [Google Scholar]