Abstract
The question of whether animals have some sort of self-awareness is a topic of continued debate. A necessary precondition for self-awareness is the ability to visually discriminate the self from others, which has traditionally been investigated through mirror self-recognition experiments. Although great apes generally pass such experiments, interpretations of results have remained controversial. The aim of this study was to investigate how bonobos (Pan paniscus) respond to different types of images of themselves and others, both before and after prolonged mirror exposure. We first presented presumably mirror-naive subjects with representations of themselves in three different ways (mirror image, contingent and non-contingent video footage) as well as representations of others (video footage of known and unknown conspecifics). We found that subjects paid significantly less attention to contingent images of themselves (mirror image, video footage) than to non-contingent images of themselves and unfamiliar individuals, suggesting they perceived the non-contingent self-images as novel. We then provided subjects with three months of access to a large mirror centrally positioned in the enclosure. Following this manipulation, subjects showed significantly reduced interest in the non-contingent self-images, while interest in unknown individuals remained unchanged, suggesting that the mirror experience has led to a fuller understanding of their own self. We discuss implications of this preliminary investigation for the on-going debate on self-awareness in animals.
Keywords: Delayed self-recognition, Mental representation, Looking time, Pan paniscus, Self-awareness, Primate cognition, Social intelligence, Intelligence, Theory of mind, Evolution of cognition
Introduction
A fundamental question in comparative cognition is whether, or to what degree, non-human animals have something akin to self-awareness, that is, whether they can recognise themselves as separate from others and the environment. Related to this is the question of whether animals, other than humans, have some understanding of their own mental states. It is generally accepted that self-awareness presupposes self-recognition of the body, such as its visual appearance, which is empirically easier to address than mentalistic notions of the self. Since the 1970s mirrors have been used as the main tool to investigate self-recognition in the visual domain. Evidence for self-recognition is either in the form of spontaneous, self-directed, exploratory behaviours to the mirror image (Swartz, Sarauw & Evans, 1999) or subjects targeting visual markings administered to a body part that is not visible without the aid of a mirror (the “mirror-mark” test; Gallup, 1970).
Gallup’s (1970) research on chimpanzees and macaques was pioneering, followed by studies on a range of other primate species, including humans (Amsterdam, 1972), chimpanzees (Lethmate & Dücker, 1973; Suárez & Gallup, 1981; Calhoun & Thompson, 1988; Swartz & Evans, 1991; Lin, Bard & Anderson, 1992; Povinelli et al., 1993), bonobos (Hyatt & Hopkins, 1994; Westergaard & Hyatt, 1994; Walraven, Van Elsacker & Verheyen, 1995), gorillas (Suárez & Gallup, 1981; Ledbetter & Basen, 1982; Posada & Colell, 2007), orang-utans (Lethmate & Dücker, 1973; Suárez & Gallup, 1981), gibbons and siamangs (Lethmate & Dücker, 1973; Inoue-Nakamura, 1997; Suddendorf & Collier-Baker, 2009), monkeys (Lethmate & Dücker, 1973; Inoue-Nakamura, 1997) and prosimians (Inoue-Nakamura, 1997) (see Anderson, 1984 for review in primates). Within the non-human primates, great apes generally appear to be capable of mirror self-recognition (see Swartz, Sarauw & Evans, 1999 for review), although there are also multiple reports of failure (e.g., gorillas: Shillito, Gallup & Beck, 1999). In bonobos, evidence for mirror self-recognition is in terms of mirror-guided, self-directed behaviours, e.g., subjects picking the teeth or eyes (Hyatt & Hopkins, 1994; Westergaard & Hyatt, 1994), in some instances from first exposure (Walraven, Van Elsacker & Verheyen, 1995). Monkeys (Macaca silenus, Mandrillus sphinx, Papio hamadryas, Ateles sp., Cebus apella) generally fail the ‘mirror-mark’ test (Lethmate & Dücker, 1973; Ujhelyi et al., 2000; Heschl & Fuchsbichler, 2009; Suddendorf & Collier-Baker, 2009) and we are not aware of any positive evidence for spontaneous, self-directed behaviours in front of mirrors (Inoue-Nakamura, 1997). In one study, Hauser et al. (1995) used a modified ‘mirror-mark’ test by colour-dying cotton-top tamarins’ (Saguinus oedipus) head hair and reported that individuals touched their heads more often and looked in the mirror longer than controls. However, the study was criticized because results were not based on blind video scoring (Anderson & Gallup, 1997). In a follow-up study, Hauser (2001) then failed to replicate the original findings but argued that subjects had witnessed other group members with colour-dyed hair, suggesting that this may have lowered their interest.
Mirror experiments have also been conducted with non-primate species, with positive evidence in bottlenose dolphins (Marten & Psarakos, 1994; Reiss & Marino, 2001), Asian elephants (Plotnik, De Waal Frans & Reiss, 2006) and even manta rays, the biggest brained of all fish (Ari & D’Agostino, 2016). At the same time, small-brained species, such as great tits (Kraft et al., 2017) or cichlid fish (Hotta, Komiyama & Kohda, 2018), typically fail mirror self-recognition tasks, suggesting that mirror self-recognition may be a property of large brains, regardless of phylogeny (but see Gallup & Anderson, 2018). At the same time, there are a number of (disputed) claims of mirror-self recognition in Clark’s nutcrackers (Clary & Kelly, 2016), Eurasian magpies (Prior, Schwarz & Güntürkün, 2008; but see Anderson & Gallup, 2015) and cleaner wrasse (Kohda et al., 2019; but see Vonk, 2020; De Waal, 2019) but not in giant pandas (Ma et al., 2015), suggesting that the complexity of a species’ social life may also play a role (Gallup, 1998; Prior, Schwarz & Güntürkün, 2008).
In humans and great apes, the capacity to recognise one’s self in a mirror emerges gradually and with experience, usually starting with social behaviours directed at the mirror (e.g., threatening or vocalising; Gallup, 1970), followed by spatial exploration (e.g., reaching or looking behind the mirror), contingency exploration (movements of mirror-image relative to subject’s body) and self-exploration (teeth, eyes or genital regions; Swartz, Sarauw & Evans, 1999). In Western human cultures, self-directed behaviours usually appear from 15–18 months of age and become fully expressed by 24 months, while photo self-recognition occurs later (e.g., Courage, Edison & Howe, 2004; Amsterdam, 1972; Lewis & Brooks-Gunn, 1979; but see Keller et al., 2004; Keller et al., 2005; Kärtner et al., 2012 for non-western cultures). In chimpanzees, early reactions to mirrors are similar in kind but do not emerge before 24 months (Lin, Bard & Anderson, 1992). Also, there are large individual differences with either much delayed onset (around 60 months: Swartz, Sarauw & Evans, 1999) or no onset at all (e.g., Swartz & Evans, 1991; Povinelli et al., 1993; Walraven, Van Elsacker & Verheyen, 1995). Mirror self-recognition studies have caused much debate on how such data should be interpreted. On one end of the spectrum is the interpretation that positive evidence is an indicator of self-awareness (Inoue-Nakamura, 1997; Gallup, 1998; Swartz, Sarauw & Evans, 1999; Plotnik, De Waal Frans & Reiss, 2006) or a self-concept (“…a sense of continuity, a sense of personal agency and a sense of identity”; Gallup, 1998, p. 240). At the other end, reactions towards administered marks have been interpreted as mere artefacts of experimental manipulations, suggesting that mirror experiments reveal nothing about cognitive capacities (Heyes, 1994; Heyes, 1995; Heyes, 1996). More intermediate positions are that such behaviour qualifies as evidence for self-perception, that is, recognising one’s own visual appearance (Nielsen, Suddendorf & Slaughter, 2006) and perhaps even that one’s own body is a separate entity from the surrounding world (a ‘body concept’). The ‘body-concept’ hypothesis has been investigated in developing children, with the conclusion that such awareness emerges in the second year of life, correlated with passing the ‘mirror-mark’ test (Moore et al., 2007). The notion of a ‘body concept’ is also key to an alternative hypothesis to self-recognition: kinaesthetic-visual matching. Here, the idea is that subjects act on the contingency between the kinaesthetic sensations (caused by their body movements) and the corresponding movements of the image in the mirror (Mitchell, 1993; Mitchell, 1997). Crucially, however, mirror self-recognition does not predict perpetual kinaesthetic-visual matching; subjects may simply look at the mirror and know that what they see is them (Mitchell, 1993).
Another way of testing self-recognition is by presenting non-contingent images, usually delayed videos or simply photographs. Infants as young as 5 months appear to discriminate between video images of themselves and those of peers or objects (Legerstee, Anderson & Schaffer, 1998) and from 18–24 months they start to show the tell-tale spontaneous behaviours, such as exploration of visually inaccessible body parts, in the absence of contingency cues (Lewis & Brooks-Gunn, 1979). Between 24 and 48 months the emergence of self-recognition from non-contingent images becomes fully established, suggesting that the task is more difficult than self-recognition in a mirror (Povinelli, Landau & Perilloux, 1996). In particular, 24–36 month-old children had more difficulties to infer the presence of a sticker on their heads from watching delayed video image of themselves than 36–48 month-old children. In a more recent study, Hirata, Fuwa & Myowa (2017) used a similar variant of the ‘mark-test’ to test five subadult chimpanzees with extensive mirror experience (three of five recognised themselves in mirrors). Here, subjects were tested with live video feedback, short-delayed video feedback (i.e., 1–4 s) and long-delayed video footage of themselves (one week). Among the control conditions, the authors used video footage of humans but not of conspecifics. The finding was that the three subjects capable of mirror self-recognition removed stickers placed on their heads more effectively and exhibited more self-directed behaviours than the other two individuals, when shown live and short-delayed video feedback but not when shown long-delayed video footage or other control conditions. Although the study lacked adequate controls (Vonk, 2018), self-recognition from delayed, non-contingent self-images may be the most stringent test that an individual (human or non-human) possesses a visual mental representation of their own appearance, and this appears to require prior experience with mirrors.
In this study, we investigated whether bonobos had a generalised understanding of their own physical appearance and whether this was dependent on experience with mirrors. To address this, we tested how subjects responded to different visual images of themselves and others, including when contingency cues were absent, and how mirror exposure influenced their performance. We hypothesised that mirror-naïve individuals did not have a full understanding of their own visual appearance, but that prolonged mirror experience could provide subjects with the crucial experience. To address this, we tested mirror-naïve subjects with different visual representations of themselves and others, including non-contingent video footage of themselves. Two years later, following a 3-month period of ad libitum access to a mirror, we retested the same subjects again with the two critical conditions, i.e., non-contingent representations of themselves and unknown others. We assessed subjects’ interest in the different stimuli by comparing looking times, based on the fact that both human and nonhuman primates spend more time looking at novel than familiar faces and other stimuli (e.g., patterns: Fantz, 1964; Gunderson & Sackett, 1984; Gunderson & Swartz, 1985; objects: Bachevalier, Brickson & Hagger, 1993; Pascalis & Bachevalier, 1998; conspecific faces: Pascalis & Bachevalier, 1998; Gothard, Erickson & Amaral, 2004; Dufour, Pascalis & Petit, 2006; Gothard, Brooks & Peterson, 2009; but see Winters, Dubuc & Higham, 2015 for criticism). We predicted that stimuli perceived as unfamiliar should cause longer looking times than stimuli perceived as familiar (Pascalis & De Schonen, 1994). Mirror self-recognition experiments do not typically test whether individuals discriminate between self and others although such a design allows for direct comparisons of attention.
Method
Study site & subjects
The study was carried out at La Vallée des Singes Primate Park in Romagne (France) with a group of bonobos (2014: N = 17; 2016: N = 20; Table 1) housed in an indoor enclosure (400 m2) with access to two outdoor wooded islands (11,500 m2). Experiments were carried out from January to July 2014 and from February to July 2016. Eight subjects (Table 1) participated in all trials, which involved a ‘looking-time’ bias task with sequential stimulus presentation, a paradigm originally developed in the late 1950s for research with pre-verbal human infants (e.g., Fantz, 1963; Winters, Dubuc & Higham, 2015).
Table 1. Study subjects housed at La Vallée des Singes primate park, France.
| Individual | Code | Sex | Age-Class | Year of birth |
|---|---|---|---|---|
| Daniela | DNL | F | Adult | 1968 |
| Lisala | LSL | F | Adult | 1980 |
| Ukela | UK | F | Adult | 1985 |
| Bondo* | BO | M | Adult | 1991 |
| Kirembo | KI | M | Adult | 1992 |
| Ulindi | UL | F | Adult | 1993 |
| Diwani | DW | M | Adult | 1996 |
| David | DV | M | Adult | 2001 |
| Khaya | KH | F | Adult | 2001 |
| Lingala | LNG | F | Sub-adult | 2003 |
| Lucy* | LY | F | Sub-adult | 2003 |
| Kelele | KEL | M | Sub-adult | 2004 |
| Luebo* | LUE | M | Sub-adult | 2006 |
| Nakala* | NK | F | Juvenile | 2007 |
| Loto | LO | M | Juvenile | 2009 |
| Moko | MO | M | Infant | 2012 |
| Khalessi | KLS | F | Infant | 2012 |
Notes.
Individuals having participated in all trials are indicated in bold (N = 8). Individuals marked by an asterisk initially participated but were excluded for reasons detailed below. Age-class as defined by Kano (1984) at beginning of study.
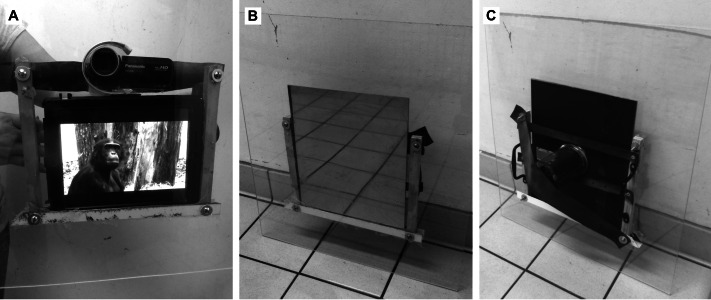
Prior to testing, individuals were exposed to the equipment during one week to minimise any potential effects of novelty. Video stimuli were then presented by means of an APPLE iPad (screen size approx. 15 × 20 cm) placed behind a transparent Acrylic panel (Fig. 1A) to which a PANASONIC HC-V100 full HD camera was mounted to record the subjects’ reactions face-on. The mirror stimulus was presented by means of a one-way mirror (25 × 30 cm), attached behind the same panel (Figs. 1B and 1C).
Figure 1. Portable acrylic panel for stimulus presentation.
(A) Video camera and iPad displaying a non-contingent video stimulus. (B) Video camera and one-way mirror (front). (C) Video camera and one-way mirror (back). Photos by Gladez Shorland.
Part 1—before mirror exposure
The first part of this within-subject experiment was carried out in 2014 with N = 8 subjects (four males, four females, age range 9–34 years). To our knowledge, subjects had no prior experience with mirrors, but we consider it likely that some individuals already had experience with reflecting surfaces, such as window glass or bodies of water. However, reflections in mirrors are of a different quality in terms of sharpness, contrast or colour accuracy, compared to reflections in other surfaces, suggesting that subjects’ experiences with reflections of their own bodies were limited.
The experiment comprised five experimental conditions presented to all subjects in the following order: (1) contingent video footage of self (live feedback of self, visible on an iPad: C self); (2) contingent mirror image of self (live depiction of self, visible in a one-way mirror: mirror); (3) non-contingent video footage of self (previously recorded video footage of self, visible on an iPad: NC self); (4) non-contingent video footage of known group member (previously recorded video footage of other group member visible on an iPad: known); (5) non-contingent video footage of unknown conspecific (previously recorded video footage of unfamiliar conspecific visible on an iPad: unknown) (see Tables S1 and S2).
Footage for ‘NC self’ and ‘known’ were recorded during the ‘mirror’ and ‘C self’ conditions. Footage for ‘unknown’ (N = 2 males; N = 1 female) were recorded at the ‘Lola ya Bonobo’ Sanctuary in the Democratic Republic of the Congo (see Movie S1 and Movie S2 for sample footage). In both the ‘known’ and ‘unknown’ conditions, the footage showed generally inactive individuals glancing at the camera from time to time. Footage for stimulus presentation was selected at random, resulting in N = 6 same-sex pairs and N = 2 opposite-sex pairs for the ‘known’ condition and N = 4 same-sex and N = 4 opposite-sex pairs for the ‘unknown’ condition. An alternative design would have been to control for sex (i.e., to compare same-sex and different-sex footage) or to simply use same-sex footage. As this would have created sample size issues we opted for randomly assigning footage of ‘known’ and ‘unknown’ individuals to our eight subjects.
Each subject was presented with each of the five conditions once and in the same sequential order over an eight-month period (see Table S2). Stimuli were presented only very briefly, for a total of 30s, starting with the first glance from the subject towards the stimulus. A trial was terminated after 30s or as soon as a subject left. We are aware of the fact that fixed order stimulus presentation designs carry the disadvantage of potentially creating cross-condition dependencies. A completely randomised design might have been preferable but not practical due to the low number of subjects available for testing. In particular, we regarded it as essential that all subjects entered part 2 of the experiment with the exact same stimulus history. We also considered it unlikely that dependencies across conditions played a role because stimulus exposure was very short (30s) and intervals between subsequent presentations were long (median = 38.5 days, range: 0–95 days).
Trials were carried out only when a subject was alone and inactive (i.e., resting or observing) in one of the indoor cages and at a suitable orientation and distance from the corridor (0.3–2.0 m). The subject was exposed to one of two portable devices, an iPad or a one-way mirror, each with a camera mounted to record looking responses (Fig. 1). From the video clips, we extracted looking-time during stimulus presentation. Looking time as a proxy for familiarity as a whole is not uncontroversial (e.g., Winters, Dubuc & Higham, 2015). In face recognition tasks, however, looking time appears to be reliable, at least for primates, who generally look longer to unfamiliar than familiar faces of conspecifics (Pascalis & De Schonen, 1994; Pascalis & Bachevalier, 1998; Gothard, Erickson & Amaral, 2004; Gothard, Brooks & Peterson, 2009; Fujita, 1987; Demaria & Thierry, 1988). Subjects’ responses were filmed with a PANASONIC HC-V100 full HD camera, as explained before, and looking-time coded post-hoc from the video recordings. Coding was blind insofar as all videos were randomly labelled so that the rater (GS) was unable to infer the experimental condition. Videos were analysed frame-by-frame with MPEG Streamclip 1.9.2. Looking time was determined by measuring the duration between the first glance towards the stimulus and the beginning of gaze aversion. If multiple gazes occurred over the 30s stimulus presentation, we added them up. All clips were coded independently and blindly by a second rater (EG), which did not reveal any reliability issues (Pearson’s correlation coefficient, r = 0.93, N = 56). Besides looking time, we were also interested in any type of mirror-guided self-directed behaviours, especially of body parts that are visually not directly accessible, such as the face, the teeth, the eyes or the back.
Eight of 17 adult group members were tested and analysed in this study (see Table 1). Regarding the remaining individuals (N = 9), one adult male (BO) participated in all experimental conditions but had to be excluded due to poor video quality that prevented accurate coding. Two further individuals (NK and LUE) also had to be excluded because they were too close to the camera, which prevented reliable coding of looking time. One adult female (LY) had to be excluded because she participated in two conditions only. Finally, we were unable to test the remaining two adult females (DNL and UK) due to their lack of interest and participation. The dependent infants and juveniles (LO, MO and KLS) were not tested.
Part 2—after mirror exposure
Fifteen months after the first part of the experiment, the same subjects were provided with prolonged access to a large mirror (45 × 115 cm) to gain extended full experience with mirror reflections of themselves. The mirror was placed in front of a resting platform in the indoor enclosure allowing extensive ad libitum access to all individuals over a period of three months (i.e., from Nov 2015 to Feb 2016; approx. 2,000 h). At this time of year, the group was kept inside due to cold weather conditions and the park was closed to the public. Although we did not quantify the amount of time and manner by which subjects interacted with the mirror, all subjects had countless opportunities to familiarise themselves with their own mirror reflections. According to the keepers, subjects did not spend noticeable amounts of time in front of the mirror during this time, nor did they notice individuals inspecting themselves in ostensive ways. In hindsight, it would have been interesting to collect systematic data on how exactly subjects behaved in front of the mirror, both quantitatively and qualitatively.
We were mainly interested in the effect of mirror experience on subjects’ perception of their own and unknown others’ non-contingent images. To this end, we retested all individuals that had participated in part 1 (N = 8) with the two critical conditions from part 1, i.e., non-contingent video footage of themselves and of unknown conspecifics. Stimulus presentation began 10–20 days after removal of the mirror. All aspects of presentation were identical to part 1, including the video footage. The time lapse between the first and second stimulus presentation for a given subject and condition was held constant for each subject and averaged at approximately 22 months (see Table S2).
Statistical analyses
In a first analysis, prior to the 3-month mirror exposure, we modelled looking time as the response variable, experimental condition as the main predictor variable and subject ID as the random intercept in a linear mixed model (LMM). We added age as a control predictor to account for the possibility that younger individuals might show more interest than older individuals (Westergaard & Hyatt, 1994; Walraven, Van Elsacker & Verheyen, 1995). Looking time and age were square root transformed to achieve homogenous and approximately normally distributed residuals. We tested this full model against an informed null model (Forstmeier & Schielzeth, 2011), which included only age as predictor and subject ID as random intercept. We tested the difference between the full and null model with a likelihood ratio test (LRT, Dobson, 2002). Note that this comparison is equivalent to testing of ‘experimental condition’ (models with vs. without experimental condition as variable). Post hoc diagnostics were implemented in order to test the stability of the model. This entailed running the full model eight times, each time removing data of one of the eight individuals and permitted us to verify whether one of the individuals was influential with respect to the interpretation of our model results. Results of this procedure indicated stable model results, that is, exclusion of any one individual did not change the conclusions of our analysis.
In a second analysis, we tested how looking time was affected by the interaction between stimulus type (self vs. unknown, i.e., self Y/N) and mirror exposure (non-exposed vs. exposed, i.e., exposed Y/N), again with a Linear Mixed Model. Once again, we controlled for age, which was included in the respective null model, and implemented post-hoc diagnostics to test the stability of the model, which returned stable model results.
Statistical analyses were carried out with R v. 3.1.2 and lme4 v. 1.1-11 (R Core Team, 2014; Bates et al., 2015). Data are available in the electronic supplementary material (Dataset S1).
Compliance with ethical standards
All applicable international, national and institutional guidelines for the care and use of animals were followed. The study was authorised and given ethical approval by the “La Vallée des Singes” scientific coordinator and zoological director. Trials were carried out opportunistically when and where subjects felt so inclined. The study was in line with the ARRIVE guidelines and recommendations from the EAZA and the AFdPZ code of ethics.
Results
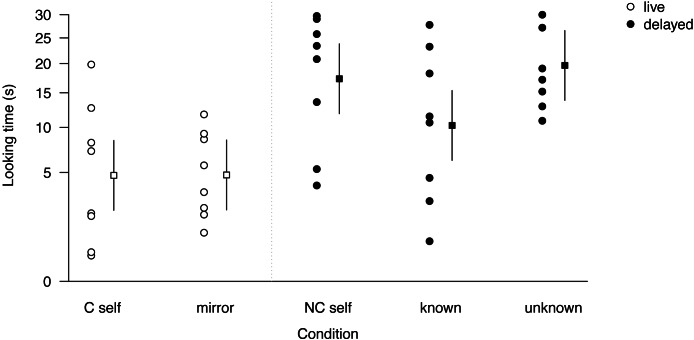
The first part of the experiment consisted of exposing subjects to different motion images of themselves, of other group members and of unfamiliar conspecifics (see Table S2). The results of the model indicated that looking time was affected by experimental condition (LRT, χ2 = 25.87, d.f. = 4, P < 0.001; Fig. 2; Table 2), with short looking times in the ‘C self’ and ‘mirror’ conditions (<5.0 of 30s) and three-fold longer looking times in the ‘NC self’ and ‘unknown’ conditions, whereas looking times to ‘known’ individuals were intermediate (Fig. 2: model estimates, Table 3: descriptive data). In other words, subjects showed most interest in images of strangers and non-contingent images of themselves and least interest in contingent images of themselves (video clips and mirror reflections), suggesting that non-contingent self-images were perceived in the same way as unknown individuals. When coding the videos for gaze duration, we did not notice any cases of self-inspecting behaviour in the two contingent conditions.
Figure 2. Subject looking time and model predictions for the five test conditions.
Subject looking time (circles) and model prediction (squares) for the five test conditions with 95% confidence interval; note that looking time was square root transformed for modeling but for presentation we back-transformed it along the y axis. Conditions are presented chronologically from left to right.
Table 2. Result of the LMM testing the effect of condition on looking time.
| Estimate | Standard error | t | |
|---|---|---|---|
| Intercept | 3.18 | 0.44 | 7.18 |
| Condition (C self) | |||
| −Mirror | 0.01 | 0.49 | 0.02 |
| −NC Self | 1.98 | 0.49 | 4.03 |
| −Known | 1.03 | 0.49 | 2.08 |
| −Unknown | 2.26 | 0.49 | 4.58 |
| Age | −0.12 | 0.03 | −3.75 |
Table 3. Descriptive results: experimental condition, mirror exposure, median looking-time and quartiles calculated from raw data.
| Condition | Mirror exposure | Median looking time | Quartiles |
|---|---|---|---|
| C self | non-exposed | 4.6 | 1.4–9.3 |
| mirror | non-exposed | 4.5 | 2.2–8.7 |
| NC self | non-exposed | 22.1 | 11.5–26.6 |
| known | non-exposed | 11.1 | 4.1–19.5 |
| unknown | non-exposed | 18.1 | 14.6–27.9 |
| NC self | exposed | 7.4 | 4.4–10.2 |
| Unknown | exposed | 18.5 | 11.5–29.7 |
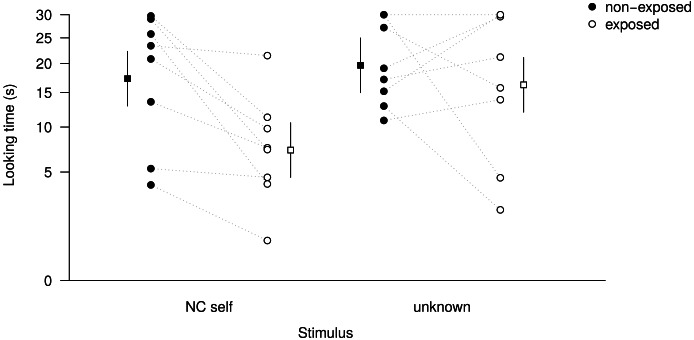
We then provided subjects with three months of ad libitum mirror access. Following this manipulation, and almost two years after the previous trials, subjects were retested with non-contingent footage of themselves (NC self) and strangers (unknown), the two conditions that elicited most interest before (Fig. 2). When comparing interest before and after mirror exposure, the full model was significantly different from the null model (LRT, χ2 = 18.06, d.f. = 3, P < 0.001; Fig. 3, Table 4), indicating that looking time differed as a function of mirror-exposure, stimulus identity, and their interaction. The interaction effect was close to significance (LRT, χ2 = 3.61, df = 1, P = 0.057), as looking time substantially decreased after mirror exposure in the ‘NC self’ condition, but not in the ‘unknown’ condition (see Fig. 3 for model prediction means and SDs). Targeted follow-up tests would be necessary to confirm the preliminary conclusion that prolonged mirror exposure decreases subjects’ interest in non-contingent self-images, while interest in stranger individuals remained unchanged.
Figure 3. Subject looking time and model predictions before and after mirror exposure.
Looking time (circles) before and after three-months ad libitum mirror exposure. Model predictions (squares) are given with 95% confidence intervals; note that looking time was square root transformed for modelling but for presentation we back-transformed it along the y axis.
Table 4. Result of the LMM, testing the effects of stimulus identity (self vs. unknown) and mirror-exposure (non-exposed vs. exposed) on looking time.
| Estimate | Standard error | t | |
|---|---|---|---|
| Intercept | 5.85 | 0.35 | 16.63 |
| Mirror-exposure (non-exposed) | |||
| - exposed | −0.40 | 0.39 | −1.04 |
| Stimulus identity (non-self) | |||
| - self | −0.27 | 0.39 | −0.71 |
| Mirror exposure: stimulus identity | −1.07 | 0.55 | −1.96 |
| Age | −0.17 | 0.03 | −6.41 |
Discussion
In this study, we were interested in how prolonged mirror-exposure influenced the response to visual representations of the self. The underlying rationale was that, during human development, recognition of non-contingent footage of the self is cognitively most challenging, suggesting that bonobos may struggle with such stimuli, more than with contingent depictions of themselves. To address this, we carried out a two-part experiment during which subjects watched motion images depicting either themselves or another individual. Due to a number of constraints, discussed below, we regard the conclusions of this study as preliminary.
In the first part, subject responses to three types of self-images (mirror reflection, contingent video footage and non-contingent video footage) were compared with responses to video footage of familiar and unfamiliar conspecifics. Results revealed low interest in the mirror and in contingent self-footage, but high interest in the non-contingent self-footage condition, similar to interest in unfamiliar individuals.
A first parsimonious explanation of this result might be that bonobos did not recognise themselves in any of the three conditions (mirror, contingent, non-contingent), but that non-contingent movement was simply more interesting than contingent movement. However, this interpretation is at odds with the fact that the ‘known’ condition caused less interest than ‘non-contingent self’ and ‘unknown’ conditions, all of which moved in asynchronous ways, so we consider this explanation as unlikely.
A second explanation for the high interest in the ‘non-contingent self’ condition might be that subjects did not recognise themselves and responded as if they were unfamiliar individuals (Fig. 2) whereas they did recognise themselves in the contingent footage and mirror images. This may be because contingent self-recognition is easier to achieve than non-contingent self-recognition, to the effect that experience with low-quality reflections (such as in windows or water surfaces) could suffice to establish some level of self-recognition.
However, if subjects recognised themselves in the contingent conditions, then why did they not show the usual tell-tale behaviours of self-recognition, such as exploration of their own teeth, nose and other visually inaccessible body parts? Possible explanations for this absence is that subjects were distracted by the experimenter or by the equipment, or that stimulus exposure was simply too short (30s) to engage in self-exploration. However, it must also be stated that during the subsequent prolonged mirror exposure, neither the keepers nor the researchers noted any self-exploration by subjects, although we did not record the subjects’ behaviour in the absence of observers.
The fact that subjects showed little interest in the contingent stimuli suggests that they were already (somewhat) familiar with their own reflections, possibly from windows, water surfaces or their own shadows, but that this was not enough to develop a full sense of how one looks. Self-recognition, in other words, may not be an all-or-nothing state in bonobos, but a gradually acquired cognitive achievement.
In the second part of the experiment, we provided subjects with extended experience of self-reflections by giving them uninterrupted access to a mirror for three consecutive months. We predicted that this experience should enable subjects to familiarise themselves with a wider range of visual depictions of themselves, which would allow them to generalise and form a mental representation of their own visual appearance. We retested subjects with only the two critical stimuli (non-contingent footage of self and unknown individuals), 10–20 days after the mirror was removed, and found interest in the non-contingent footage significantly decreased, while interest in strangers remained similar (Fig. 3). In hindsight, a further interesting comparison would have been to also rerun the ‘known’ condition as a control. Based on the fact that the subjects had extensive opportunities to observe each other daily, we would not have predicted any change in interest in this condition after the mirror-exposure.
Could results be explained by low-level stimulus habituation to differences in background? We find this an unlikely scenario since stimulus presentations were exceedingly short (30s) and presented twice only over a period of 22 months, rendering perceptual habituation an implausible explanation. Related to this, subjects could have paid more attention to some backgrounds than others. Although we cannot rule this out completely, the looking time data are at odds with such an explanation: Despite similar backgrounds, responses to non-contingent self and known individuals were different, while responses to non-contingent self and strangers were similar, despite different backgrounds.
As it stands, our results are thus consistent with two hypotheses, i.e., that subjects responded to differences in perceived familiarity or that they recognized some faces as their own. At the very least, therefore, subjects must have managed to familiarise themselves with all aspects of their visual appearance, allowing them to categorise the ‘non-contingent self’ footage as more familiar compared to the stranger footage. But whether subjects really proceeded to form a true mental concept of their own visual appearance (“that’s me”), as opposed to some lower level sense of familiarity, will have to be addressed by future research. For instance, subjects could be shown (ideally contingent) footage of the stranger individuals to control for the extra time they were granted to watch themselves in the mirror. For example, subjects could be shown footage of the stranger individuals interacting with the mirror images, but such that the filmed mirror reflection only revealed their faces. If despite this extra experience they continued to show higher interest in the non-contingent footage of strangers, familiarity is the less likely explanation, suggesting that subjects understood that the individual in the video clip was them.
Conclusion
Our data suggest that, given sufficient mirror exposure, bonobos acquire the ability to use mirror-reflections of themselves to learn about their own physical appearance in more generalised way. In contrast to other research, our results suggest that this level of awareness can be detached from the here and now and can include visual non-contingent representations of the self. Whether this was achieved by responding to differences in perceived familiarity or full self-recognition cannot be conclusively decided by our investigation. Much remains to be elucidated regarding the mechanisms and the implications of great apes’ capacity of self-recognition, such as the amount of mirror exposure minimally necessary or the stability of the resulting self-recognition over time (see Calhoun & Thompson, 1988; De Veer et al., 2003).
Supplemental Information
Acknowledgments
We thank the keepers, Carole Michelet, Franck Alexieff, Lise Morel, Jérémy Mergault and Alexandre Albert for their valuable help in carrying out the experiment. Many thanks to Christof Neumann for his statistical advice and for valuable discussions and support.
Funding Statement
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement n° 283871 and the Swiss National Science Foundation (Social learning in primate communication: 31003A_166458 / Coordinating joint action in apes: Testing the boundaries of the human interaction engine: CR31I3_159655). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Gladez Shorland conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Emilie Genty conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jean-Pascal Guéry performed the experiments, authored or reviewed drafts of the paper, coordinated access to study animals and gave advice on all aspects of the study, and approved the final draft.
Klaus Zuberbühler conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This experimental study was authorised and given ethical approval by the “La Vallée des Singes” scientific coordinator and zoological director.
Data Availability
The following information was supplied regarding data availability:
The raw dataset is available in the Supplementary File.
The video recordings of the experimental trials are provided on figshare: Shorland, Gladez; Genty, Emilie; Guéry, Jean-Pascal; Zuberbühler, Klaus (2020): Trial recordings : N=8 individuals x 7 conditions. Figshare. Media. https://doi.org/10.6084/m9.figshare.12608786.v1.
References
- Amsterdam (1972).Amsterdam B. Mirror self-image reactions before age two. Developmental Psychobiology. 1972;5:297–305. doi: 10.1002/dev.420050403. [DOI] [PubMed] [Google Scholar]
- Anderson (1984).Anderson JR. Monkeys with mirrors: some questions for primate psychology. International Journal of Primatology. 1984;5:81–98. doi: 10.1007/BF02735149. [DOI] [Google Scholar]
- Anderson & Gallup (2015).Anderson JR, Gallup GG. Mirror self-recognition: a review and critique of attempts to promote and engineer self-recognition in primates. Primates. 2015;56:317–326. doi: 10.1007/s10329-015-0488-9. [DOI] [PubMed] [Google Scholar]
- Anderson & Gallup (1997).Anderson J, Gallup JG. Self-recognition in Saguinus? A critical essay. Animal Behavior. 1997;54:1559–1563. doi: 10.1006/anbe.1997.0548. [DOI] [PubMed] [Google Scholar]
- Ari & D’Agostino (2016).Ari C, D’Agostino DP. Contingency checking and self-directed behaviors in giant manta rays: do elasmobranchs have self-awareness? Journal of Ethology. 2016;34:167–174. doi: 10.1007/s10164-016-0462-z. [DOI] [Google Scholar]
- Bachevalier, Brickson & Hagger (1993).Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. NeuroReport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Bates et al. (2015).Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Calhoun & Thompson (1988).Calhoun S, Thompson RL. Long-term retention of self-recognition by chimpanzees. American Journal of Primatology. 1988;15:361–365. doi: 10.1002/ajp.1350150409. [DOI] [PubMed] [Google Scholar]
- Clary & Kelly (2016).Clary D, Kelly DM. Graded mirror self-recognition by Clark’s nutcrackers. Scientific Reports. 2016;6:36459. doi: 10.1038/srep36459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courage, Edison & Howe (2004).Courage ML, Edison SC, Howe ML. Variability in the early development of visual self-recognition. Infant Behavior & Development. 2004;27(4):509–532. doi: 10.1016/j.infbeh.2004.06.001. [DOI] [Google Scholar]
- De Veer et al. (2003).De Veer MW, Gallup GG, Theall LA, Van den Bos R, Povinelli DJ. An 8-year longitudinal study of mirror self-recognition in chimpanzees (Pan troglodytes) Neuropsychologia. 2003;41:229–234. doi: 10.1016/S0028-3932(02)00153-7. [DOI] [PubMed] [Google Scholar]
- De Waal (2019).De Waal FBM. Fish, mirrors, and a gradualist perspective on self-awareness. PLOS Biology. 2019;17(2):e3000112. doi: 10.1371/journal.pbio.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria & Thierry (1988).Demaria C, Thierry B. Responses to animal stimulus photographs in stumptailed macaques (Macaca arctoides) Primates. 1988;29:237–244. doi: 10.1007/BF02381125. [DOI] [Google Scholar]
- Dobson (2002).Dobson AJ. An introduction to generalized linear models. Chapman & Hall/CRC; Boca Raton: 2002. [Google Scholar]
- Dufour, Pascalis & Petit (2006).Dufour V, Pascalis O, Petit O. Face processing limitation to own species in primates: a comparative study in brown capuchins, Tonkean macaques and humans. Behavioural Processes. 2006;73:107–113. doi: 10.1016/j.beproc.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Fantz (1963).Fantz RL. Pattern vision in newborn infants. Science. 1963;140:296–297. doi: 10.1126/science.140.3564.296. [DOI] [PubMed] [Google Scholar]
- Fantz (1964).Fantz RL. Visual experiences in infants: decreased attention to familiar patterns relative to novel ones. Science. 1964;146:668–670. doi: 10.1126/science.146.3644.668. [DOI] [PubMed] [Google Scholar]
- Forstmeier & Schielzeth (2011).Forstmeier W, Schielzeth H. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behavioral Ecology and Sociobiology. 2011;65:47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita (1987).Fujita K. Species recognition by five macaque monkeys. Primates. 1987;28:353–366. doi: 10.1007/BF02381018. [DOI] [Google Scholar]
- Gallup (1970).Gallup GG. Chimpanzees: self-recognition. Science. 1970;167:86–87. doi: 10.1126/science.167.3914.86. [DOI] [PubMed] [Google Scholar]
- Gallup (1998).Gallup GG. Self-awareness and the evolution of social intelligence. Behavioural Processes. 1998;42:239–247. doi: 10.1016/S0376-6357(97)00079-X. [DOI] [PubMed] [Google Scholar]
- Gallup & Anderson (2018).Gallup GG, Anderson JR. The “olfactory mirror” and other recent attempts to demonstrate self-recognition in non-primate species. Behavioural Processes. 2018;148:16–19. doi: 10.1016/j.beproc.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Gothard, Brooks & Peterson (2009).Gothard KM, Brooks KN, Peterson MA. Multiple perceptual strategies used by macaque monkeys for face recognition. Animal Cognition. 2009;12:155–167. doi: 10.1007/s10071-008-0179-7. [DOI] [PubMed] [Google Scholar]
- Gothard, Erickson & Amaral (2004).Gothard KM, Erickson CA, Amaral DG. How do rhesus monkeys (Macaca mulatta) scan faces in a visual paired comparison task? Animal Cognition. 2004;7:25–36. doi: 10.1007/s10071-003-0179-6. [DOI] [PubMed] [Google Scholar]
- Gunderson & Sackett (1984).Gunderson VM, Sackett GP. Development of pattern recognition in infant pigtailed macaques (Macaca nemestrina) Developmental Psychology. 1984;20:418–426. doi: 10.1037/0012-1649.20.3.418. [DOI] [Google Scholar]
- Gunderson & Swartz (1985).Gunderson VM, Swartz KB. Visual recognition in infant pigtailed macaques after a 24-hour delay. American Journal of Primatology. 1985;8:259–264. doi: 10.1002/ajp.1350080309. [DOI] [PubMed] [Google Scholar]
- Hauser (2001).Hauser MD. Cotton-top tamarins fail to show mirror-guided self-exploration. American Journal of Primatology. 2001;53:131–137. doi: 10.1002/1098-2345(200103)53:3<131::AID-AJP4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hauser et al. (1995).Hauser MD, Kralik J, Bottomahan C, Garrett M, Oser J. Self-recognition in primates—phylogeny and the salience of species-typical features. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(23):10811–10814. doi: 10.1073/pnas.92.23.10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heschl & Fuchsbichler (2009).Heschl A, Fuchsbichler C. Siamangs (Hylobates syndactylus) recognize their mirror image. International Journal of Comparative Psychology. 2009;22:221–233. [Google Scholar]
- Heyes (1994).Heyes CM. Reflections on self-recognition in primates. Animal Behaviour. 1994;47:909–919. doi: 10.1006/anbe.1994.1123. [DOI] [Google Scholar]
- Heyes (1995).Heyes CM. Self-recognition in primates: further reflections create a hall of mirrors. Animal Behaviour. 1995;50:1533–1542. doi: 10.1016/0003-3472(95)80009-3. [DOI] [Google Scholar]
- Heyes (1996).Heyes CM. Self-recognition in primates: irreverence, irrelevance and irony. Animal Behaviour. 1996;51:470–473. doi: 10.1006/anbe.1996.0048. [DOI] [Google Scholar]
- Hirata, Fuwa & Myowa (2017).Hirata S, Fuwa K, Myowa M. Chimpanzees recognize their own delayed self-image. Royal Society Open Science. 2017;4:1–9. doi: 10.1098/rsos.170370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta, Komiyama & Kohda (2018).Hotta T, Komiyama S, Kohda M. A social cichlid fish failed to pass the mark test. Animal Cognition. 2018;21:127–136. doi: 10.1007/s10071-017-1146-y. [DOI] [PubMed] [Google Scholar]
- Hyatt & Hopkins (1994).Hyatt CW, Hopkins WD. Self-awareness in bonobos and chimpanzees: a comparative perspective. In: Parker ST, Mitchell RW, Boccia ML, editors. Self-awareness in animals and humans: developmental perspectives. Cambridge University Press; New York: 1994. pp. 248–253. [Google Scholar]
- Inoue-Nakamura (1997).Inoue-Nakamura N. Mirror self-recognition in nonhuman primates: a phylogenetic approach. Japanese Psychological Research. 1997;39:266–275. doi: 10.1111/1468-5884.00059. [DOI] [Google Scholar]
- Kano (1984).Kano T. Distribution of pygmy chimpanzees (Pan paniscus) in the central Zaire basin. Folia Primatologica. 1984;43(1):36–52. doi: 10.1159/000156169. [DOI] [Google Scholar]
- Kärtner et al. (2012).Kärtner J, Keller H, Chaudhary N, Yovsi R. The development of mirror self-recognition in different sociocultural contexts. Monographs of the Society for Research in Child Development. 2012;77(4):i-101. doi: 10.1111/j.1540-5834.2012.00688.x. [DOI] [PubMed] [Google Scholar]
- Keller et al. (2005).Keller H, Kärtner J, Borke J, Yovsi R, Kleis A. Parenting styles and the development of the categorical self: A longitudinal study on mirror self-recognition in Cameroonian Nso and German families. International Journal of Behavioral Development. 2005;29:496–504. doi: 10.1080/01650250500147485. [DOI] [Google Scholar]
- Keller et al. (2004).Keller H, Yovsi R, Borke J, Kartner J, Jensen H, Papaligoura Z. Developmental consequences of early parenting experiences: self-recognition and self-regulation in three cultural communities. Child Development. 2004;75:1745–1760. doi: 10.1111/j.1467-8624.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- Kohda et al. (2019).Kohda M, Hotta T, Takeyama T, Awata S, Tanaka H, Asai JY, Jordan AL. If a fish can pass the mark test, what are the implications for consciousness and self-awareness testing in animals? PLOS Biology. 2019;17(2):e3000021. doi: 10.1371/journal.pbio.3000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft et al. (2017).Kraft F-L, Forštová T, Utku Urhan A, Exnerová A, Brodin A. No evidence for self-recognition in a small passerine, the great tit (Parus major) judged from the mark/mirror test. Animal Cognition. 2017;20:1049–1057. doi: 10.1007/s10071-017-1121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter & Basen (1982).Ledbetter DH, Basen JA. Failure to demonstrate self-recognition in gorillas. American Journal of Primatology. 1982;2:307–310. doi: 10.1002/ajp.1350020309. [DOI] [PubMed] [Google Scholar]
- Legerstee, Anderson & Schaffer (1998).Legerstee M, Anderson D, Schaffer A. Five- and eight-month-old infants recognize their faces and voices as familiar and social stimuli. Child Development. 1998;69:37–50. doi: 10.2307/1132068. [DOI] [PubMed] [Google Scholar]
- Lethmate & Dücker (1973).Lethmate J, Dücker G. Untersuchungen zum selbsterkennen im spiegel bei orang-utans und einigen anderen affenarten. Ethology. 1973;33:248–269. [PubMed] [Google Scholar]
- Lewis & Brooks-Gunn (1979).Lewis M, Brooks-Gunn J. Social cognition and the acquisition of self. Springer US; Boston: 1979. [Google Scholar]
- Lin, Bard & Anderson (1992).Lin AC, Bard KA, Anderson JR. Development of self-recognition in chimpanzees (Pan troglodytes) Journal of Comparative Psychology. 1992;106:120–127. doi: 10.1037/0735-7036.106.2.120. [DOI] [PubMed] [Google Scholar]
- Ma et al. (2015).Ma X, Jin Y, Luo B, Zhang G, Wei R, Liu D. Giant pandas failed to show mirror self-recognition. Animal Cognition. 2015;18:713–721. doi: 10.1007/s10071-015-0838-4. [DOI] [PubMed] [Google Scholar]
- Marten & Psarakos (1994).Marten K, Psarakos S. Evidence of self-awareness in the bottlenose dolphin (Tursiops truncatus) In: Parker ST, Mitchell RW, Boccia ML, editors. Self-awareness in animals and humans: developmental perspectives. Cambridge University Press; New York: 1994. pp. 361–379. [Google Scholar]
- Mitchell (1993).Mitchell RW. Mental models of mirror-self-recognition: two theories. New Ideas in Psychology. 1993;11:295–325. doi: 10.1016/0732-118X(93)90002-U. [DOI] [Google Scholar]
- Mitchell (1997).Mitchell RW. Kinesthetic-visual matching and the self-concept as explanations of mirror-self-recognition. Journal for the Theory of Social Behaviour. 1997;27:17–39. doi: 10.1111/1468-5914.00024. [DOI] [Google Scholar]
- Moore et al. (2007).Moore C, Moore C, Mealiea J, Garon N, Povinelli DJ. The development of body self-awareness. Infancy. 2007;11:157–174. doi: 10.1111/j.1532-7078.2007.tb00220.x. [DOI] [Google Scholar]
- Nielsen, Suddendorf & Slaughter (2006).Nielsen M, Suddendorf T, Slaughter V. Mirror self-recognition beyond the face. Child Development. 2006;77:176–185. doi: 10.1111/j.1467-8624.2006.00863.x. [DOI] [PubMed] [Google Scholar]
- Pascalis & Bachevalier (1998).Pascalis O, Bachevalier J. Face recognition in primates: a cross-species study. Behavioural Processes. 1998;43:87–96. doi: 10.1016/S0376-6357(97)00090-9. [DOI] [PubMed] [Google Scholar]
- Pascalis & De Schonen (1994).Pascalis O, De Schonen S. Recognition memory in 3- to 4-day-old human neonates. NeuroReport. 1994;5:1721–1724. doi: 10.1097/00001756-199409080-00008. [DOI] [PubMed] [Google Scholar]
- Plotnik, De Waal Frans & Reiss (2006).Plotnik JM, De Waal Frans BM, Reiss D. Self-recognition in an Asian elephant. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17053–17057. doi: 10.1073/pnas.0608062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada & Colell (2007).Posada S, Colell M. Another gorilla (Gorilla gorilla gorilla) recognizes himself in a mirror. American Journal of Primatology. 2007;69:576–583. doi: 10.1002/ajp.20355. [DOI] [PubMed] [Google Scholar]
- Povinelli, Landau & Perilloux (1996).Povinelli DJ, Landau KR, Perilloux HK. Self-recognition in young children using delayed versus live feedback: evidence of a developmental asynchrony. Child Development. 1996;67:1540–1554. doi: 10.2307/1131717. [DOI] [PubMed] [Google Scholar]
- Povinelli et al. (1993).Povinelli DJ, Rulf AB, Landau KR, Bierschwale DT. Self-recognition in chimpanzees (Pan troglodytes): distribution, ontogeny, and patterns of emergence. Journal of Comparative Psychology. 1993;107:347–372. doi: 10.1037/0735-7036.107.4.347. [DOI] [PubMed] [Google Scholar]
- Prior, Schwarz & Güntürkün (2008).Prior H, Schwarz A, Güntürkün O. Mirror-induced behavior in the magpie (Pica pica): evidence of self-recognition. PLOS Biology. 2008;6:e202. doi: 10.1371/journal.pbio.0060202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2014).R Core Team . Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Reiss & Marino (2001).Reiss D, Marino L. Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5937–5942. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillito, Gallup & Beck (1999).Shillito D, Gallup JG, Beck B. Factors affecting mirror behavior in western lowland gorillas, Gorilla gorilla. Animal Behavior. 1999;57:999–1004. doi: 10.1006/anbe.1998.1062. [DOI] [PubMed] [Google Scholar]
- Suárez & Gallup (1981).Suárez SD, Gallup GG. Self-recognition in chimpanzees and orangutans, but not gorillas. Journal of Human Evolution. 1981;10:175–188. doi: 10.1016/S0047-2484(81)80016-4. [DOI] [Google Scholar]
- Suddendorf & Collier-Baker (2009).Suddendorf T, Collier-Baker E. The evolution of primate visual self-recognition: evidence of absence in lesser apes. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1671–1677. doi: 10.1098/rspb.2008.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz & Evans (1991).Swartz KB, Evans S. Not all chimpanzees (Pan troglodytes) show self-recognition. Primates. 1991;32:483–496. doi: 10.1007/BF02381939. [DOI] [Google Scholar]
- Swartz, Sarauw & Evans (1999).Swartz KB, Sarauw D, Evans S. Comparative aspects of mirror self-recognition in great apes. In: Parker ST, Mitchell RW, Miles HL, editors. The mentalities of gorillas and orangutans: comparative perspectives. Cambridge University Press; Cambridge: 1999. pp. 283–294. [Google Scholar]
- Ujhelyi et al. (2000).Ujhelyi M, Merker B, Buk P, Geissmann T. Observations on the behavior of gibbons (Hylobates leucogenys, H. gabriellae, and H. lar) in the presence of mirrors. Journal of Comparative Psychology. 2000;114:253–262. doi: 10.1037//0735-7036.114.3.253. [DOI] [PubMed] [Google Scholar]
- Vonk (2018).Vonk J. Are chimpanzees stuck on their selves in video? Learning & Behavior. 2018;46(3):227–228. doi: 10.3758/s13420-018-0328-z. [DOI] [PubMed] [Google Scholar]
- Vonk (2020).Vonk J. A fish eye view of the mirror test. Learning & Behavior. 2020;48(2):193–194. doi: 10.3758/s13420-019-00385-6. [DOI] [PubMed] [Google Scholar]
- Walraven, Van Elsacker & Verheyen (1995).Walraven V, Van Elsacker L, Verheyen R. Reactions of a group of pygmy chimpanzees (Pan paniscus) to their mirror-images: evidence of self-recognition. Primates. 1995;36:145–150. doi: 10.1007/BF02381922. [DOI] [Google Scholar]
- Westergaard & Hyatt (1994).Westergaard GC, Hyatt CW. The responses of bonobos (Pan paniscus) to their mirror images: evidence of self-recognition. Human Evolution. 1994;9:273–279. doi: 10.1007/BF02435514. [DOI] [Google Scholar]
- Winters, Dubuc & Higham (2015).Winters S, Dubuc C, Higham JP. Perspectives: the looking time experimental paradigm in studies of animal visual perception and cognition. Ethology. 2015;121:625–640. doi: 10.1111/eth.12378. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw dataset is available in the Supplementary File.
The video recordings of the experimental trials are provided on figshare: Shorland, Gladez; Genty, Emilie; Guéry, Jean-Pascal; Zuberbühler, Klaus (2020): Trial recordings : N=8 individuals x 7 conditions. Figshare. Media. https://doi.org/10.6084/m9.figshare.12608786.v1.