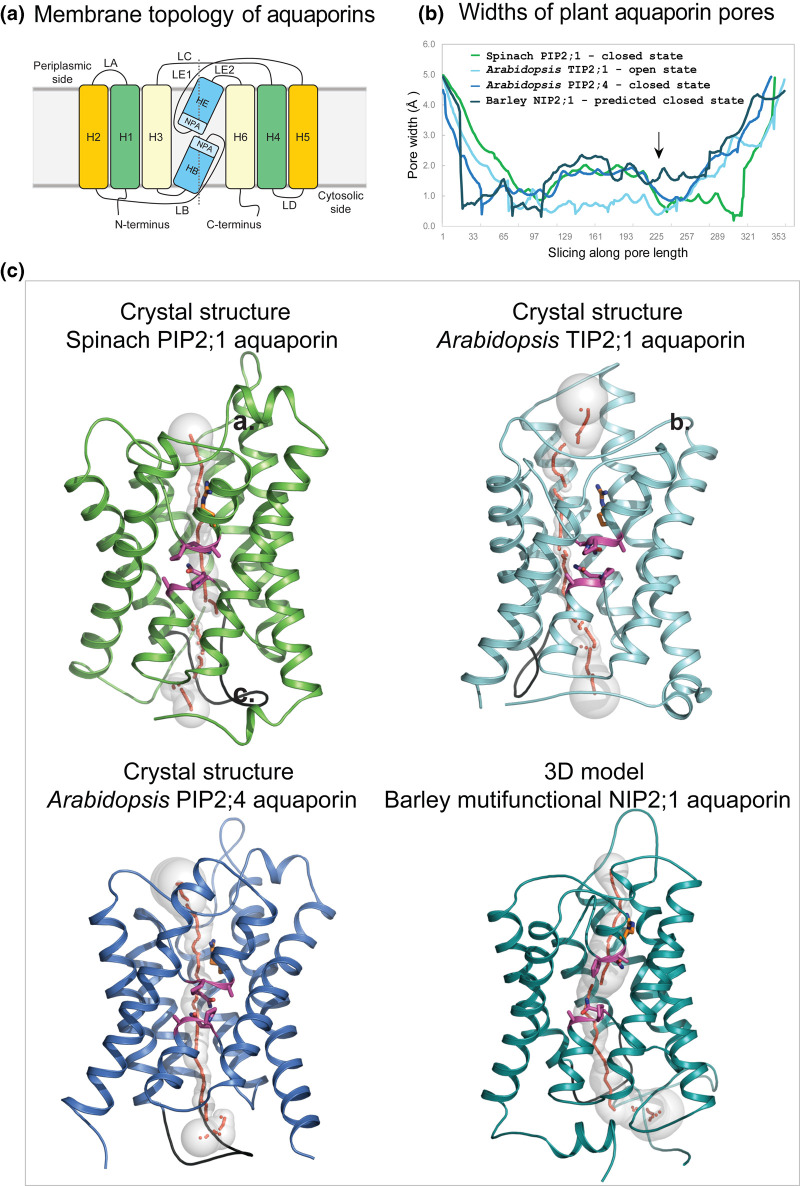
Figure 2. Molecular structures of plant aquaporins involved in water, metalloid, ion and other solute transport.
(a) Membrane topology diagram of aquaporins. Each aquaporin molecule consists of six H1–H6 transmembrane α-helices (orange, green, pale yellow boxes) and two re-entrant HB and HE α-helices (marine). Transmembrane α-helices are inter-connected via five LA–LE loops. Two Asn-Pro-Ala (NPA) motifs HE (flanked by LE1 and LE2 loops) and HB (flanked by LB loop) (aquamarine) are separated by ∼4–5 Å. The dotted line separates bipartite structural repeats of hour-glass folded aquaporins. (b) Profiles of pore width parameters of aquaporins (in Å) calculated by Hole [91] and conformational states (closed or open) of spinach PIP2;1 (PDB 1z98; green), and Arabidopsis TIP2;1 (PDB 5i32; cyan) and PIP2;4 (PDB 6qim; deep blue) aquaporins. The 3D model of barley NIP2;1 (teal) was built by trRosetta [38] as described in the text. Barley NIP2;1 has a wider pore (arrow) along its entire length, compared with those of predominantly water-permeable aquaporins. (c) Cartoon representations of monomeric aquaporins (colouring as described above) enclose pores with morphologies shown by sequences of grey spheres (pore radii are equal to sphere diameters) and red dots. Two conserved NPA motifs (magenta sticks) (cf. panel (a)) and neighbouring Arg222 (one of the selectivity filter residues, orange sticks) line pores in central regions. LD loops (black) show similar dispositions in all aquaporins and are assumed to be essential for pore closure at cytosolic sides of proteins. In barley NIP2;1 the last 20 residues were omitted for clarity.