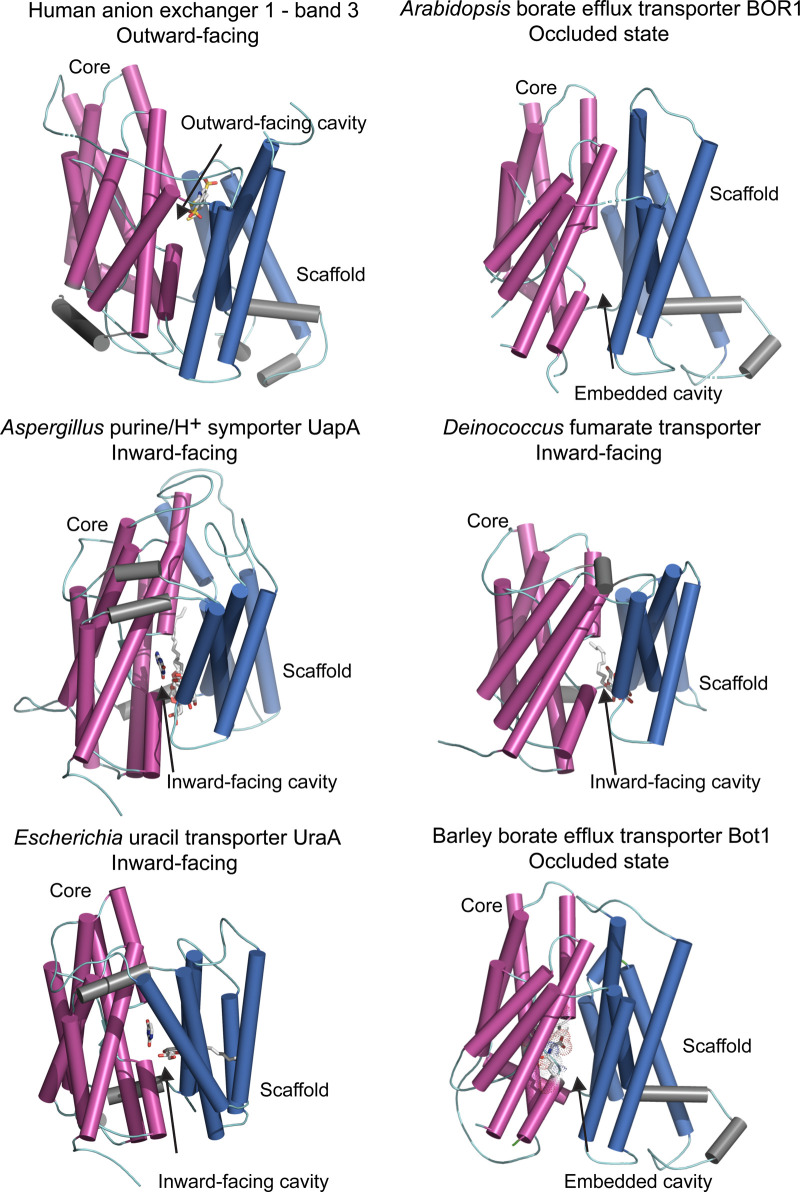
Figure 3. Molecular structures of the elevator-type transporters that permeate a variety of solutes.
Cartoon representations of monomeric efflux transporters with membrane cylindrical α-helices indicating conformational states. Cartoons of human anion exchanger — band 3 (PDB 4yzf), Arabidopsis BOR1 borate efflux transporter (PDB 5l25), Aspergillus UapA purine/H+ symporter (PDB 5i6c), Deinococcus fumarate transporter (PDB 5da0) and Escherichia UraA uracil transporter (PDB 3qe7) illustrate dispositions of core (magenta) relative to those of scaffold (gate) (deep blue) domains, and additional α-helices (grey). Arrows indicate dispositions of outward- and inward-facing or embedded cavities containing bound solutes (grey sticks). UapA, fumarate and UraA structures contain dodecyl-β-d-maltoside surfactant, n-nonyl-β-d-maltoside surfactant, and n-nonyl-β-d-glucoside surfactant and the pyrimidine ring of uracil, respectively (grey sticks). 3D model (based on the 5l25 template) of the barley efflux transporter Bot1 is included for comparison, where the longest intervening loops adjoining cylindrical α-helices were omitted for clarity. CPK sticks with dots, illustrating Van der Waals radii indicate the positions of highly conserved Ala96, Asp317, Asn361 and Gln366 residues that are likely to be involved in the permeation of [B(OH)4]− by barley Bot1.