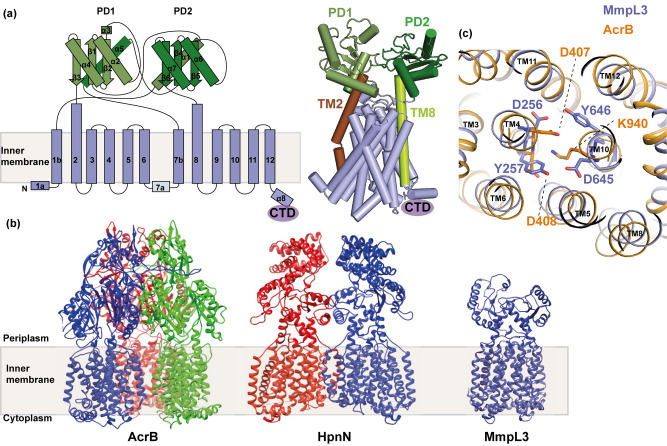
Figure 2. Structural comparison of MmpL3 with other bacterial RND transporter proteins.
(a) Secondary structural topology and crystal structure of MmpL3773 (PDB ID: 6N40) monomer viewed in the membrane plane. The TMs are labelled from 1–12, periplasmic domains are highlighted as PD1 and PD2 and C-terminal domain as CTD. Protrusion of TM2 and TM8 into the periplasm is highlighted in the structure. (b) Comparison of MmpL3 3D topology with the existing structures of bacterial RND transporters. (c) Superposition of MmpL3 TM domains (blue) with AcrB (gold). The Asp-Tyr dyads, which are important for proton relay network of MmpL3 are located in a similar position to that of D-D-K triad of AcrB.