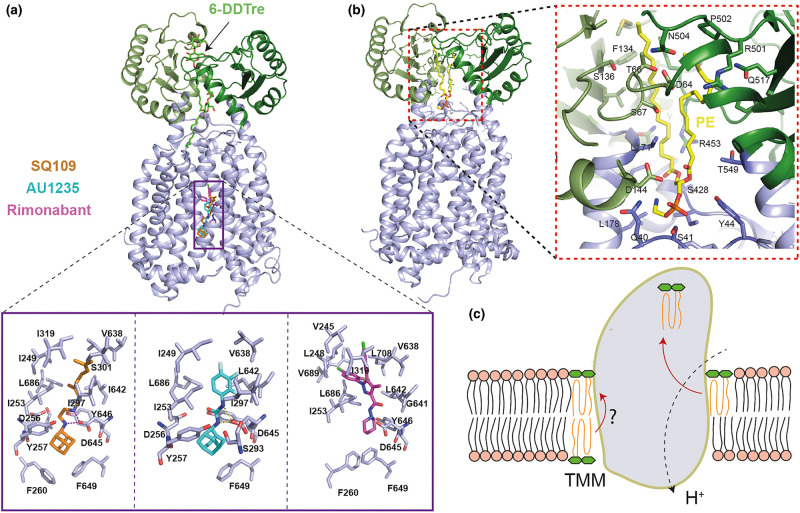
Figure 3. Analysis of the inhibitor binding and PE binding pockets provide insights into the mechanism of action of MmpL3 inhibitors and TMM transport mechanism, respectively.
(a) Crystal structures of MmpL3748 with inhibitors (PDB ID: 6AJG, 6AJH, 6AJI). The two 6-DDTre molecules are shown in light blue sticks and bound inhibitors (SQ109 in gold, AU1235 in cyan and Rimonabant in pink) from three different structures are overlaid and shown together for simplicity (PDB ID: 1IWG, 5KHS, 6N40). A close-up view of the binding site of these inhibitors shows the disruption of the interaction between Asp-Tyr pairs involved in proton translocation. (b) Crystal structure of MmpL3773 in complex with PE (PDB ID: 6OR2) and close up view of PE binding pocket. (c) Schematic illustration of proposed MmpL3-mediated TMM transport. Two putative substrate entry pathways are shown. One from the inner leaflet to outer leaflet through TM groove, consistent with its flippase activity; the other is located at the outer leaflet of the inner membrane, which then gets transported to the binding site (as evident from PE binding and 6-DDTre binding) in the PD domain for subsequent release.