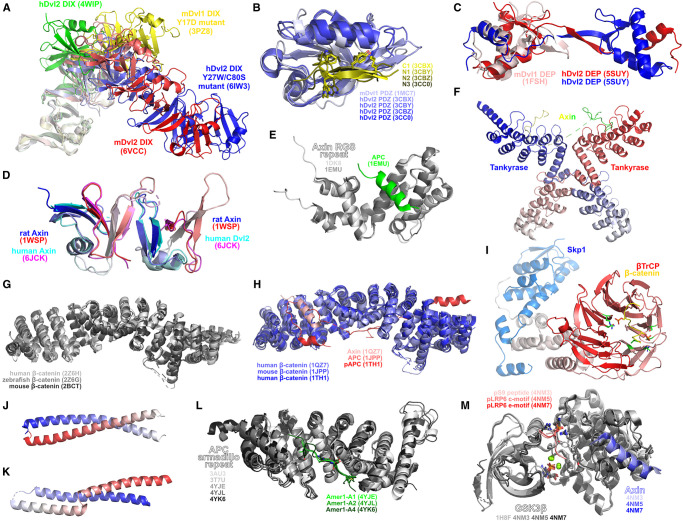
Figure 5. Structures related to cytoplasmic proteins and interactions.
(A) Dvl DIX domain oligomeric structures. (B) Dvl PDZ domain. Structures represented include: native mouse Dvl1 PDZ (PDB 1MC7), human Dvl2 PDZ in complex with C1 inhibitory peptide (PDB 3CBX), human Dvl2 PDZ in complex with N1 inhibitory peptide (PDB 3CBY), human Dvl2 PDZ in complex with N2 inhibitory peptide (PDB 3CBZ), human Dvl2 PDZ in complex with N3 inhibitory peptide (PDB 3CC0). The structure of the N2 complex with Dvl2 PDZ was inferred via the generation of a symmetry-related dimer. Selected residues with similar chemical functionality across multiple peptides — loosely highlighting how Fzd KTxxxW motifs may be recognised by Dvl PDZ domains — are shown as sticks. (C) Dvl DEP domain. Structures represented include: native mouse Dvl1 DEP domain (PDB 1FSH); human Dvl2 DEP domain dimer crystallised from dimeric fraction (PDB 5SUY). (D) Axin DIX domain. Structures represented include: rat Axin homodimer (PDB 1WSP); human Axin-Dvl2 heterodimer (PDB 6JCK). Each molecule coloured from N-to-C-terminal in blue-to-red/cyan-to-magenta gradient. (E) Axin RGS repeat in unbound (PDB 1DKS) and APC-bound (PDB 1EMU) states. (F) Mouse tankyrase-axin complex (PDB 3UTM). Each tankyrase monomer is coloured from N-to-C terminal as blue/red to white gradient. Dashes indicate missing portions of the axin structure. (G) Native β-catenin structures. (H) Complexes of interactions of cytoplasmic β-catenin. Structures represented include: human β-catenin bound to Xenopus Axin (PDB 1QZ7); mouse β-catenin bound to an APC fragment (PDB 1JPP); human β-catenin bound to a phosphorylated human APC fragment (PDB 1TH1). (I) APC N-terminal coiled-coil region (residues 2–55) (PDB 1DEB). Each chain coloured from N-to-C terminal as blue/red to white gradient. (J) APC N-terminal helical region (residues 126–250) (PDB 1M51). Coloured from N-to-C terminal as blue-white-red gradient. (K) APC armadillo repeat. Structures represented include: native APC (PDB 3AU3 and 3T7U); APC in complex with Amer1-A1 (PDB 4YJE); APC in complex with Amer1-A2 (PDB 4YJL); APC in complex with Amer1-A4 (PDB 4YK6). (L) β-TrCP-Skp1-β-catenin complex (PDB 1P22). Phosphate-contacting residues in β-TrCP are shown green. Phosphorylated β-catenin residues and their contacts in β-TrCP shown as sticks. (M) GSK3β complexes elaborating Wnt signalling. Structures represented include: apo-GSK3β (PDB 1H8F); GSK3β bound to N-terminal autoinhibitory phosphopeptide (pS9) and Axin (PDB 4NM3); GSK3β bound to phosphorylated LRP6 c-motif and Axin (PDB 4NM5); GSK3β bound to phosphorylated LRP6 e-motif and Axin (PDB 4NM7). Bound ADP, phosphorylated residues on peptides and Arg96, Arg180, Lys205 and Tyr216 shown as sticks. Magnesium shown as green spheres.