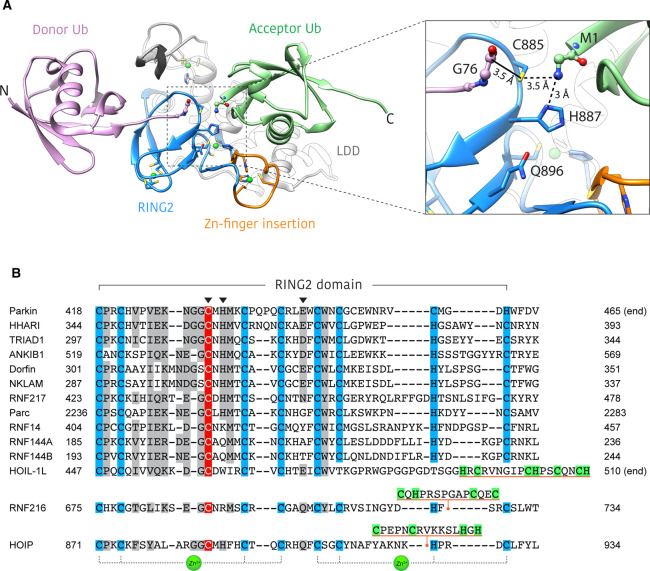
Figure 3. Structural basis for ubiquitin linkage specificity in HOIP.
(A) Structure of HOIP RING2-LDD/ubiquitin complex (PDB: 4LJO) [22]. The HOIP RING2-LDD (blue and white) orients the donor ubiquitin (purple) and acceptor ubiquitin (green) in such a way as to align the N and C-termini for M1 linkage formation. The unique RING2 zinc-finger insert (orange) forms part of the acceptor ubiquitin binding platform. Inset shows an enlarged view of the active site, highlighting the proximity of donor ubiquitin C-terminus, RING2 active site cysteine and the acceptor ubiquitin M1-amino group. The solid black line represents the E3∼Ub thioester bond that is not present in this structure. Residues of the RING2 catalytic triad are shown as blue sticks. (B) Alignment of human RBR RING2 domain sequences. Conserved zinc coordinating residues = blue, conservatively replaced residues = grey, active site cysteine = red. Catalytic triad residues are denoted by an arrowhead. Unique RING2 inserts for HOIP, HOIL-1L and RNF216 are underlined orange, with putative zinc binding residues highlighted green. The last three zinc coordinating residues of the HOIL-1L RING2 are not aligned with other family members, as the identity of these residues is currently unknown.