Fig. 3. In situ–produced bFGF has greater bioactivity than recombinantly produced bFGF.
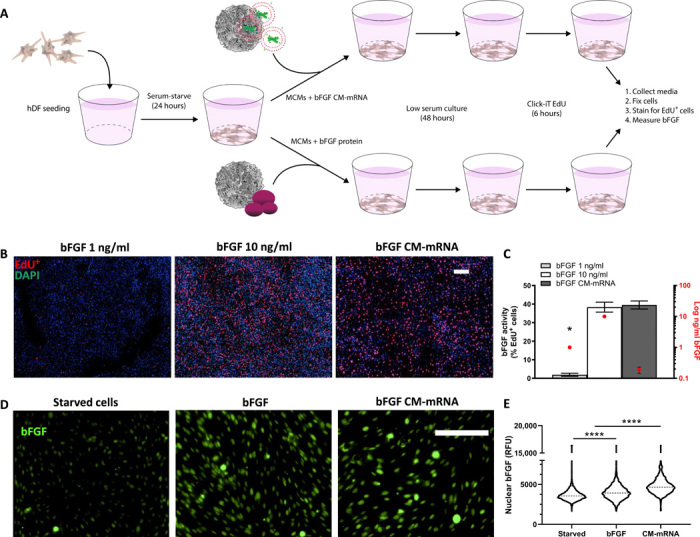
(A) Schematic of in situ recombinantly produced bFGF bioactivity comparison. Serum-starved hDFs were treated with recombinant bFGF (1 or 10 ng/ml) or bFGF CM-mRNA, all via MCMs. Forty-eight hours after treatment, the media was collected for bFGF quantification via enzyme-linked immunosorbent assay and cells analyzed for proliferation. (B) Representative epifluorescent micrographs of 1 (left) and 10 ng/ml (middle) recombinant bFGF and 300-ng CM-mRNA (right) all delivered with MCMs. DAPI+ nuclei (blue) and S phase+ nuclei (red). Scale bar, 100 μm. (C) Number of S phase+ nuclei as a percentage of total nuclei (left axis) and amount of total bFGF delivered or produced (right axis, log scale). n = 3; *P < 0.0001 by one-way ANOVA with Tukey’s post hoc analysis. (D) Right: Representative epifluorescence micrographs for nuclear bFGF immunofluorescence in serum-starved hDF treated with bFGF (10 ng/ml), bFGF CM-mRNA, or no treatment. Scale bar, 100 μm. (E) Image cytometry of nuclear immunofluorescence for bFGF in serum-starved hDF treated with bFGF (10 ng/ml), bFGF CM-mRNA, or no treatment. Nuclei (1500 to 2500) measured, pooled from two experimental replicates. ****P < 0.0001 by one-way ANOVA with Tukey’s post hoc analysis for multiple comparisons. RFU, relative fluorescence units.
