Fig. 2. Exogenous hydrogen sulfide supplementation either by chemical donors or CBS overexpression rescued hypoxic stress.
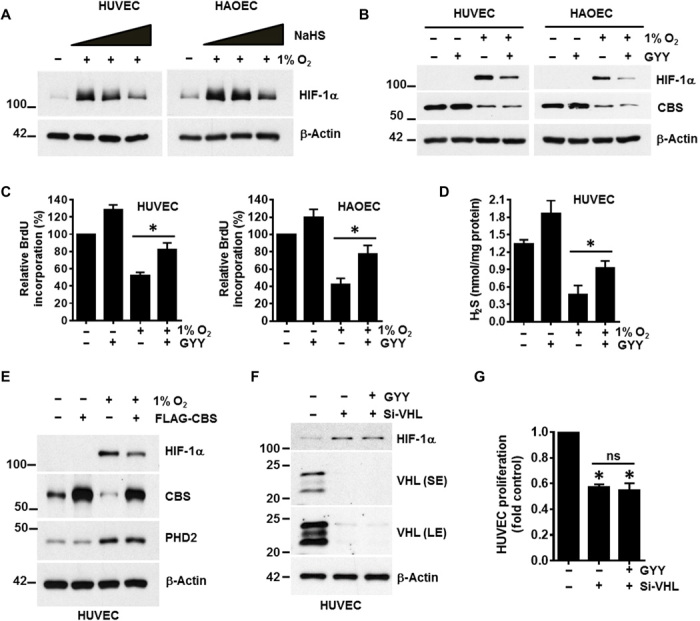
(A) HUVEC and HAOEC were exposed to normoxia or hypoxia (1% O2) for 48 hours; for the final 2 hours, ECs were exposed to various concentrations (0.1, 0.5, and 1 mM) of an H2S donor, NaHS. Expression of HIF-1α and β-actin was determined by immunoblotting. (B) HUVEC and HAOEC were exposed to normoxia or 1% O2 in the presence or absence of 0.5 mM of another small-molecule slow-release H2S generator, GYY4137 (GYY) for 48 hours, and immunoblotting were performed with the respective indicated antibodies. (C) HUVEC and HAOEC were exposed to normoxia or 1% O2 in the presence or absence of 0.5 mM GYY for 48 hours, and cellular proliferation was assessed using the BrdU assay. Data are the means ± SD of three independent experiments performed in triplicate. *P < 0.05 when comparing the specified groups. (D) HUVEC was exposed to normoxia or 1% O2 in the presence or absence of 0.5 mM GYY for 48 hours, and H2S levels were determined by the methylene blue assay. Data are the means ± SD of three independent experiments performed in triplicate. *P < 0.05 when comparing with respective untreated normoxic controls by one-way ANOVA. (E) HUVECs were first transfected with either empty vector or the FLAG-CBS construct and then further exposed to normoxia or 1% O2 for 48 hours before determining the expression of HIF-1α, CBS, PHD2, and β-actin by immunoblotting. (F) HUVEC was transfected with either scrambled or VHL siRNA (si-VHL) and was supplemented with or without 0.5 mM GYY for 48 hours, and immunoblotting was performed with the indicated antibodies. SE and LE represent as shorter exposure and longer exposure, respectively, of VHL blots during development. (G) Both si-Con– and si-VHL–transfected HUVEC were supplemented with 0.5 mM GYY for 48 hours, and proliferation was evaluated using the CyQUANT Assay. Data are the means ± SD of three independent experiments performed in triplicate. *P < 0.05 when compared with the si-Control by a one-way ANOVA. ns, not significant.
