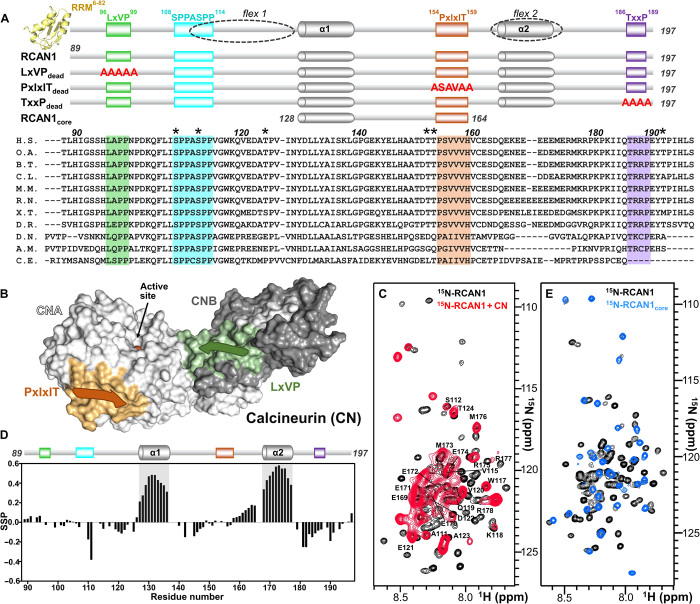
Fig. 1. RCAN1 interacts extensively with CN.
(A) RCAN1 domain structure showing the N-terminal RRM domain (yellow, amino acids 6 to 82; PDB ID 1WEY), the LxVP motif (green, amino acids 96 to 99), the SPPASPP motif (cyan, amino acids 108 to 114), the PxIxIT motif (orange, amino acids 154 to 159), and the TxxP motif (purple, amino acids 186 to 189). Partially populated helices α1 and α2 [gray cylinders; see (D)] and the regions that remain flexible when bound to CN [dotted circles; flex1 and flex2; see (C)] are also indicated. Constructs used in this study are shown below, with mutations shown in red letters. Sequence alignment of multiple RCAN1 species (amino acids 89 to 197), with the residues corresponding to motifs highlighted; * indicates residues phosphorylated by active p38α. RCAN1 orthologs used in sequence alignment are described in Materials and Methods. RCAN1 residue numbers are labeled according to human RCAN1 isoform 2. (B) Cartoon diagram of calcineurin (CN), with CNA shown as a white surface, CNB shown as a gray surface, the PxIxIT motif–binding pocket shown in orange, the LxVP motif–binding pocket shown in green, and the location of the active site indicated. (C) Overlay of the 2D [1H,15N] HSQC spectra of 15N-labeled RCAN1 in the absence (black) and presence (red) of CN. Peaks that do not interact directly with CN are labeled. (D) Secondary-structure propensity data plotted against RCAN1 residue numbers. These data indicate regions with transient secondary structure (SSP > 0, α helix; SSP < 0, β strand). (E) Overlay of the 2D [1H,15N] HSQC spectra of 15N-labeled RCAN1 (black) and 15N-labeled RCAN1core (blue).